Therapeutic and prophylactic methods for neuromuscular disorders
a neuromuscular disorder and prophylactic method technology, applied in the field of clinical pathophysiology, can solve the problems of abnormal sarcolemmal membrane tearing, no effective arresting of the disease course, and many forms of muscular dystrophies that are fatal and currently incurable, so as to reduce the prednisone-induced muscle atrophy and increase muscle mass and strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Effect of GDF-8 Neutralizing Antibody on Dystrophic Muscle
[0057]The ability of in vivo inhibition of GDF-8 to ameliorate muscular dystrophy was tested in the mdx mouse model of DMD. Five to seven week old male C57BL / 10ScSn-mdx / j mice (Jackson Laboratory, Bar Harbor, Me.) were treated with weekly intraperitoneal injections of the GDF-8 neutralizing murine antibody JA-16 (60 mg / kg, double dosing at first week, n=11), and vehicle alone (control group, n=10) for 12 weeks. These mice were also compared to mice of the same background strain (C57BL / 10, n=12) without the dystrophin deficiency.
[0058]The body weight was monitored before, during and after treatment. Mice in the treatment group gained weight relative to mice in the vehicle control group. Results are shown in Table 1.
TABLE 1Total body weight (g) Average values with SEMWeekJA-16vehicle controlvehicle controlof trial(mdx)(mdx)(non-mdx)021.92 + / − 0.4222.51 + / − 0.3619.18 + / − 0.40427.82 + / − 0.4326.76 + / − 0.6024.14 + / − 0.27829.59 + / − ...
example 2
Effect of GDF-8 Neutralizing Antibody and Prednisone on Normal and Dystrophic Muscle
[0061]Male C57BL / 10ScSn-mdx / j and C57BL / 10 (Jackson Laboratory, Bar Harbor, Me.). Mouse monoclonal anti-GDF-8 antibody JA-16, prednisone (P-9901, Sigma), or vehicle (peanut oil) was injected starting at age 5-7 weeks for 4 weeks. Mice were intraperitoneally (IP) injected with JA-16 at a dose of 60 mg / kg per week (double dosing at first week), or subcutaneously (SC) injected with prednisone at 2 mg / kg, 3 times a week.
[0062]The body weight and grip strength were monitored before, during and after treatment. Results are shown in Table 3 and Table 4, respectively.
TABLE 3Total body weight (average ± SEM, g)WeekPrednisone +vehiclevehicleofJA-16Prednisonecontrolcontroltrial(mdx)(mdx)(mdx)(non-mdx)017.7 ± 1.617.6 ± 1.816.0 ± 1.919.2 ± 0.4122.1 ± 1.420.9 ± 1.819.1 ± 2.122.4 ± 0.3225.9 ± 1.224.2 ± 1.623.7 ± 1.423.8 ± 0.4326.5 ± 1.124.7 ± 1.524.8 ± 1.224.9 ± 0.4428.1 ± 1.225.9 ± 1.625.7 ± 1.425.6 ± 0.5
TABLE 4Gr...
example 3
Effect of GDF-8 Neutralizing Antibody on Prednisone-Induced Muscle Atrophy
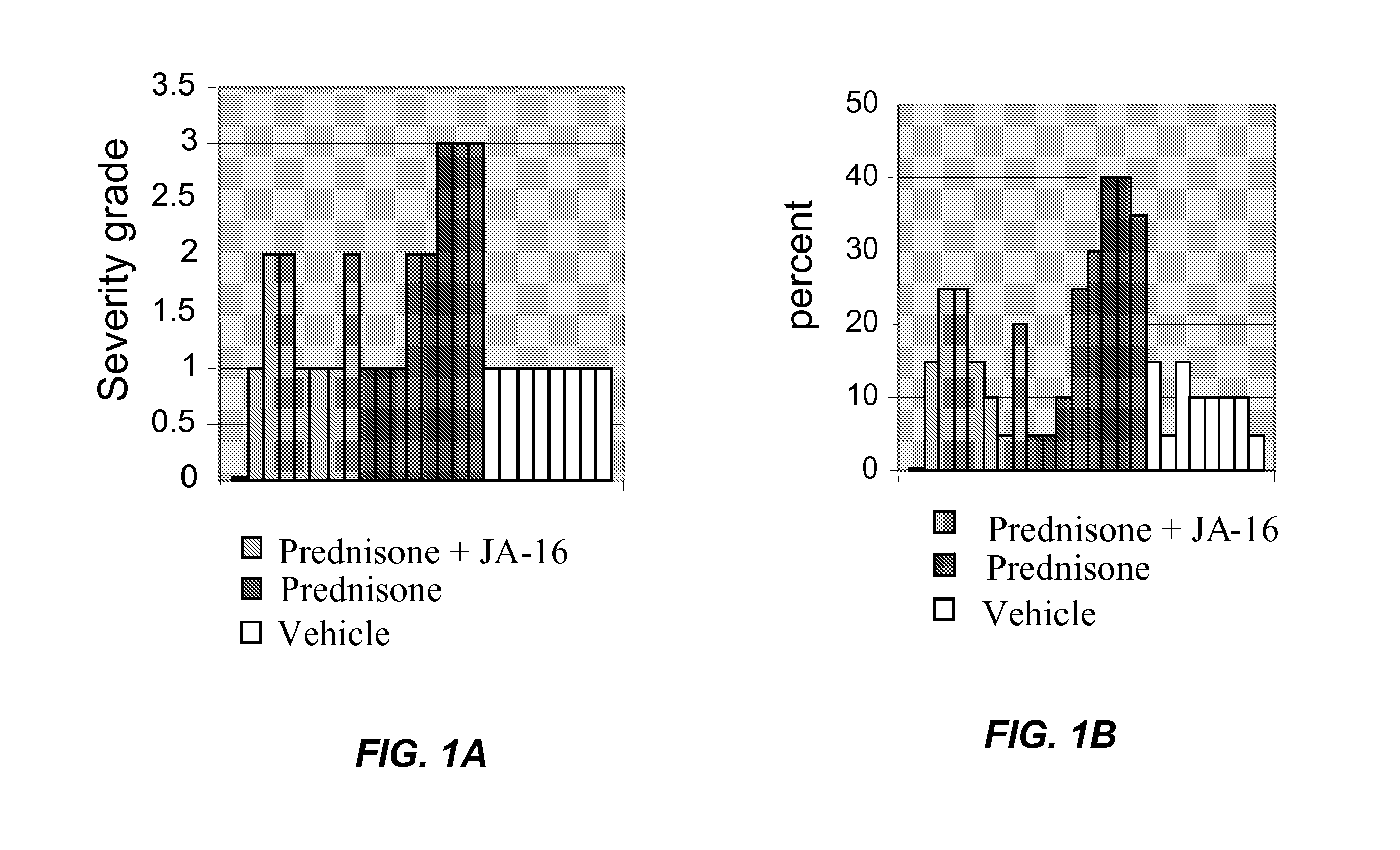
[0066]In the mice treated as described in Example 2, diaphragm muscle was histologically examined as described in Example 1. The morphological changes were evaluated by an independent pathology lab that had no knowledge of the treatment group assignments. Severity grades were assigned on a scale from 0 to 4 (0=none; 1=minimal; 2=mild; 3=moderate; and 4=marked). Results are shown in FIG. 1A (severity scores) and FIG. 1B (percentage of muscle fibers atrophied). The results show that administration of the anti-GDF-8 antibody with prednisone reduces prednisone-induced muscle atrophy.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com