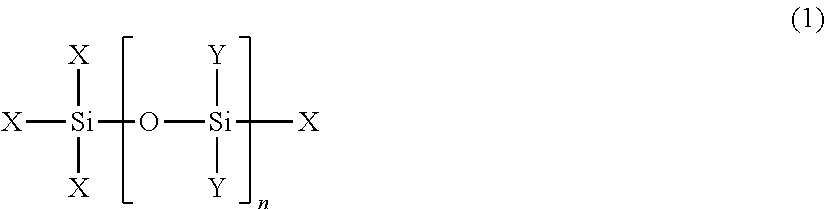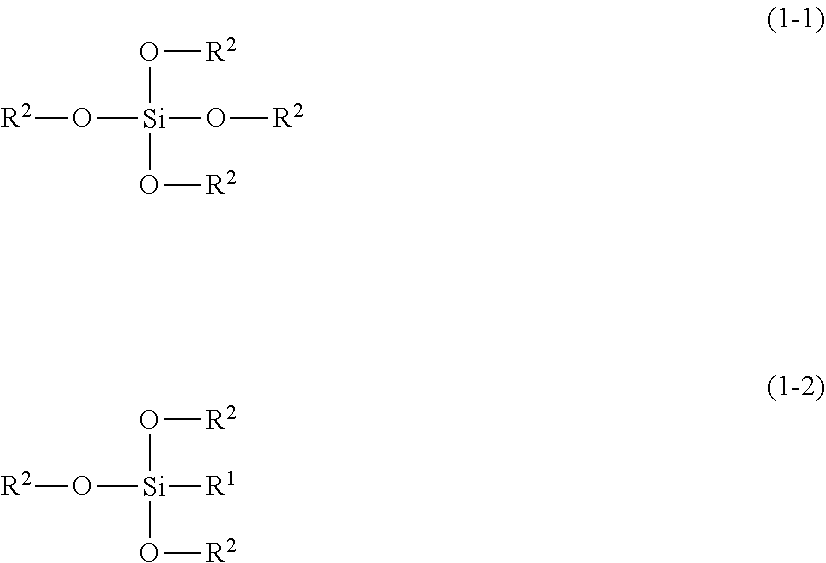Textile treatment composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
Synthesis of octylsilicic Acid tris(2-phenylethyl)ester [tris(2-phenylethyloxy)octylsilane]
[0095]In a 300 mL four-neck flask, under a nitrogen flow, 83.01 g of octyltriethoxysilane (0.30 mol), 127.76 g of phenylethyl alcohol (0.83 mol), and 0.857 mL of a solution of 2.8% sodium methoxide in methanol were stirred for 2.5 hours at 110 to 115° C., while distilling ethanol off. After 2.5 hours, the inner pressure of the reaction vessel was gradually reduced to 8 kPa. The mixture was stirred for additional 3 hours at 110 to 119° C. with distilling ethanol off. After 3 hours, the mixture was cooled and the reduced pressure was released. The mixture was filtered to give 173.61 g of yellow oil containing octylsilicic acid tris(2-phenylethyl) ester.
synthesis example 2
Synthesis of silicic Acid tetrakis(cis-3-hexenyl)ester [tetrakis(cis-3-hexenyloxy)silane]
[0096]In a 200 mL four-neck flask, under a nitrogen flow, 35.45 g of tetraethoxysilane (0.17 mol), 64.74 g of cis-3-hexenol (0.65 mol), and 1.34 mL of a solution of 2.8% sodium methoxide in methanol were stirred for about 2 hours at 118 to 120° C. with distilling ethanol off. After 2 hours, the inner pressure of the reaction vessel was gradually reduced to 8 kPa. The mixture was stirred for additional 3 hours at 112 to 119° C. with distilling ethanol off. After 3 hours, the mixture was cooled and the reduced pressure was released. The mixture was filtered to give 66.17 g of light brown oil containing tetrakis(cis-3-hexenyloxy)silane.
synthesis example 3
Synthesis of poly(4-methoxyphenylmethoxy)siloxane
[0097]In a 100 mL four-neck flask, under a nitrogen flow, 72.96 g of tetraethoxysilane, 0.24 g of potassium hydroxide, and 0.4 mL of ion-exchanged water were reacted for about 37 hours at 120 to 125° C. and 33 kPa to 101 kPa (ambient pressure). During the reaction, 0.4 mL of ion-exchanged water was further added. The reaction was continued for additional 2 hours at 33 kPa. The mixture was cooled and filtered to produce 67.29 g of ethoxysilane condensate as a light yellow liquid.
[0098]Then, in a 100 mL four-neck flask, 25.00 g of the tetraethoxysilane condensate obtained above, 56.39 g of 4-methoxyphenylmethanol, and 0.17 g of a 4.8% aqueous solution of sodium hydroxide were stirred for 2 hours at 95 to 119° C. with distilling ethanol off. After 2 hours, the inner pressure of the reaction vessel was gradually reduced to 8 kPa. The mixture was stirred for additional 3 hours at 116 to 119° C. with distilling ethanol off. After 3 hours, t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com