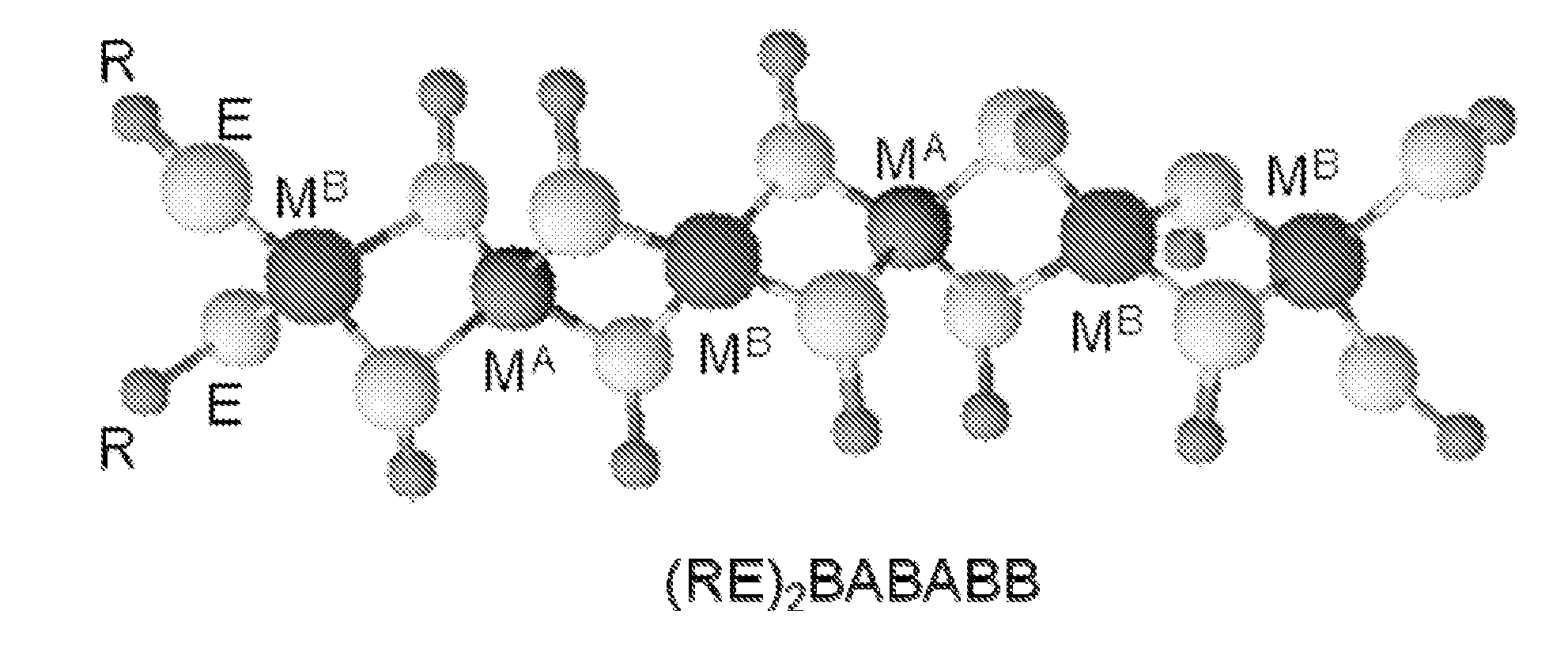
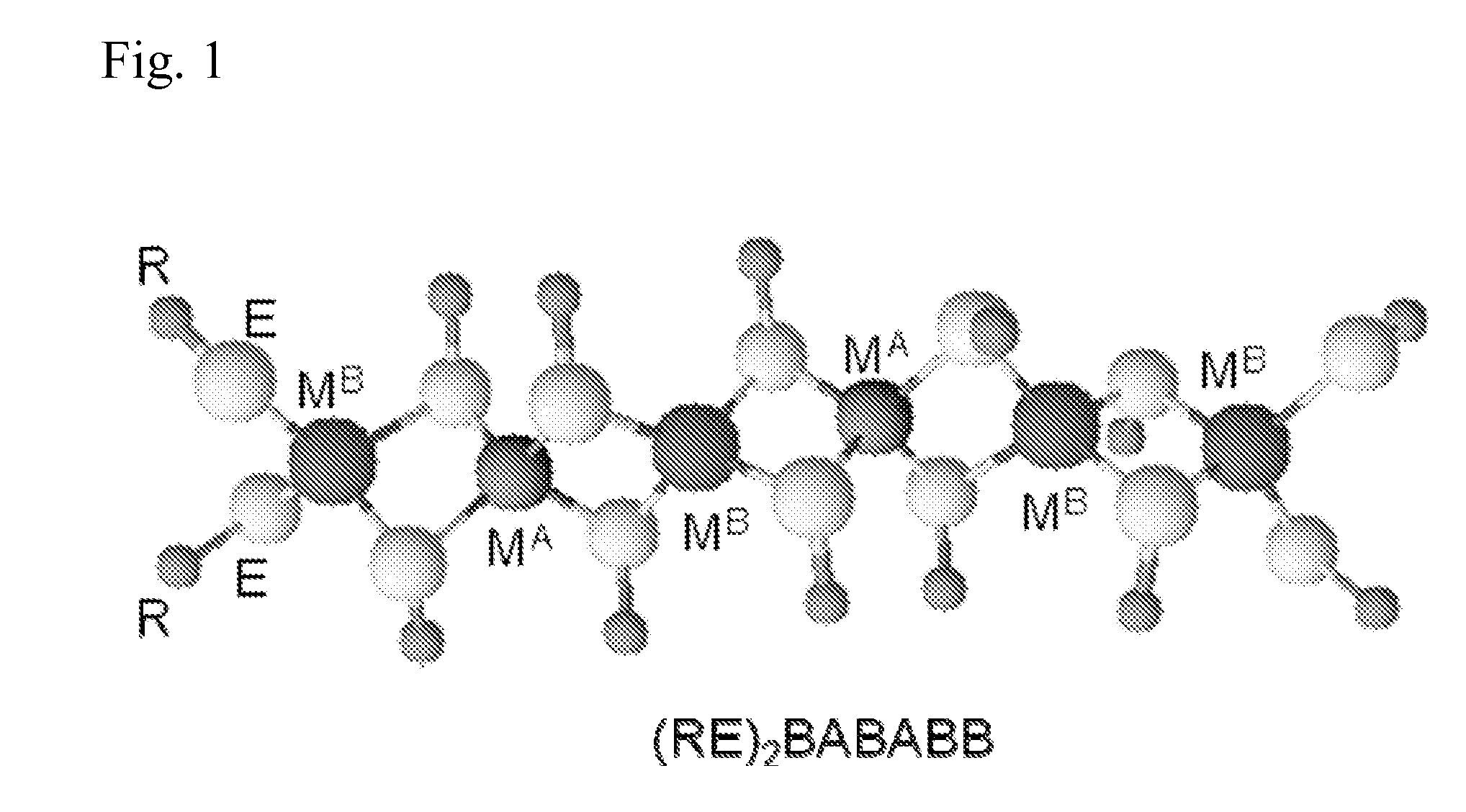
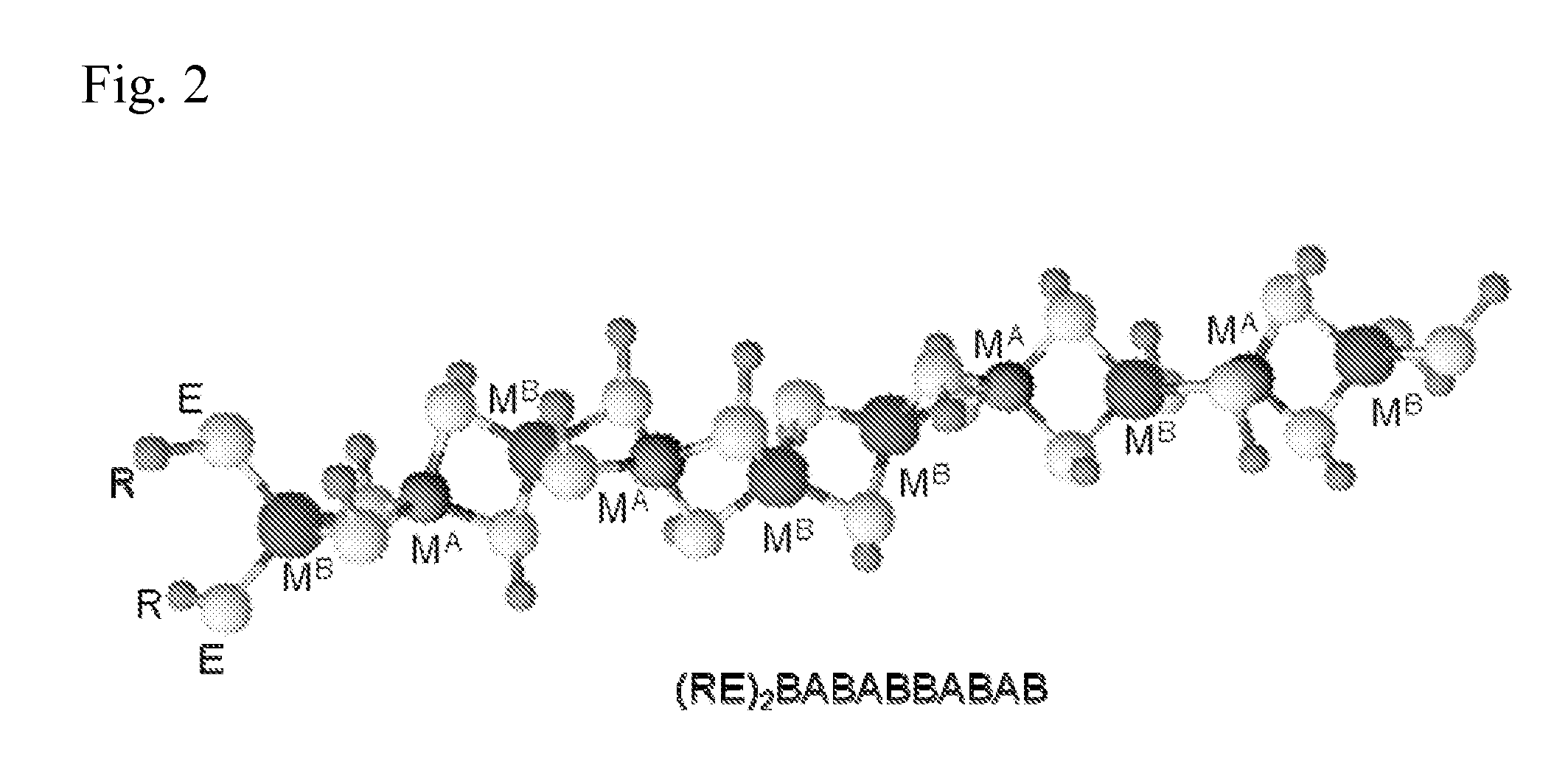
Polymeric precursors for aigs silver-containing photovoltaics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Polymeric Precursor Compounds and Compositions
[0565]A polymeric precursor represented by the formula {Cu0.5Ag0.5(SesBu)4In} was synthesized using the following procedure.
[0566]In an inert atmosphere glovebox, a Schlenk tube was charged with 0.64 g (1.22 mmol) of In(SesBu)3, 0.15 g (0.61 mmol) of AgSesBu and 0.12 g (0.60 mmol) of CuSesBu. Benzene (15 mL) was added, and the reaction mixture was stirred at 25° C. for 30 min. An orange solution was obtained. This solution was filtered through a filter cannula and the mother liquor was collected. The solvent was then removed under dynamic vacuum. 0.88 g (97%) of orange solid was recovered.
[0567]Elemental analysis: C, 25.3, H, 4.61. NMR: (1H) 1.03 (br), 1.72 (br), 1.80 (br), 2.05 (br), 3.72 (br), 3.95 (br) in C6D6.
[0568]The TGA for this MPP-CAIGS polymeric precursor showed a transition beginning at about 146° C., having a midpoint at about 211° C., and ending at 220° C. The yield for the transition was 49.6% (w / w), as compa...
example 2
[0569]A polymeric precursor represented by the formula {Cu0.7Ag0.1(SesBu)3.8Ga0.3In0.7} was synthesized using the following procedure.
[0570]In an inert atmosphere glovebox, a Schlenk tube was charged with 0.74 g (1.4 mmol) of In(SesBu)3, 0.28 g (0.59 mmol) of Ga(SesBu)3, 0.28 g (1.4 mmol) of CuSesBu and 48 mg (0.2 mmol) of AgSesBu. Benzene (15 mL) was added, and the reaction mixture was stirred at 25° C. for 30 min. An orange solution was obtained. This solution was filtered through a filter cannula and the mother liquor was collected. The solvent was then removed under dynamic vacuum. 1.27 g (94%) of orange oil was recovered.
[0571]Elemental analysis: C, 26.8, H, 4.04. NMR: (1H) 1.02 (br), 1.65 (br), 1.84 (br), 2.02 (br), 3.71 (br) in C6D6.
[0572]The TGA for this MPP-CAIGS polymeric precursor showed a transition beginning at about 146° C., having a midpoint at about 212° C., and ending at 235° C. The yield for the transition was 51.0% (w / w), as compared to a theoretical yield for the...
example 3
[0573]A polymeric precursor represented by the formula {Cu0.8Ag0.2(SesBu)4In} was synthesized using the following procedure.
[0574]In an inert atmosphere glovebox, a Schlenk tube was charged with 0.52 g (1.0 mmol) of In(SesBu)3, 0.16 g (0.8 mmol) of CuSesBu and 49 mg (0.2 mmol) of AgSesBu. Benzene (15 mL) was added, and the reaction mixture was stirred at 25° C. for 30 min. An orange solution was obtained. This solution was filtered through a filter cannula and the mother liquor was collected. The solvent was then removed under dynamic vacuum. 0.72 g (99%) of orange oil was recovered.
[0575]Elemental analysis: C, 26.32, H, 4.87, In, 14.34, Ag, 1.54, Cu, 7.94. NMR: (1H) 0.99 (br), 1.11 (br), 1.71 (br), 1.81 (br), 2.03 (br), 3.68 (br) in C6D6.
[0576]In FIG. 8 is shown the TGA for this MPP-CAIGS polymeric precursor. The TGA showed a transition beginning at about 139° C., having a midpoint at about 212° C., and ending at 225° C. The yield for the transition was 46.2% (w / w), as compared to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Band gap | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com