Methods and Compositions for Treating Polyps
a technology for nasal polyps and compositions, applied in the field of methods and compositions for treating nasal polyps, can solve the problems of reducing the productivity and affecting the quality of life of affected individuals, and affecting the treatment of nasal polyps with antibiotics, steroids, allergy shots, etc., and achieves the effect of increasing the gene transcription of a target and the expression of a target protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
Example 1
Gene Expression Profiling of Nasal Polyps Associated with Chronic Sinusitis and Aspirin-Sensitive Asthma
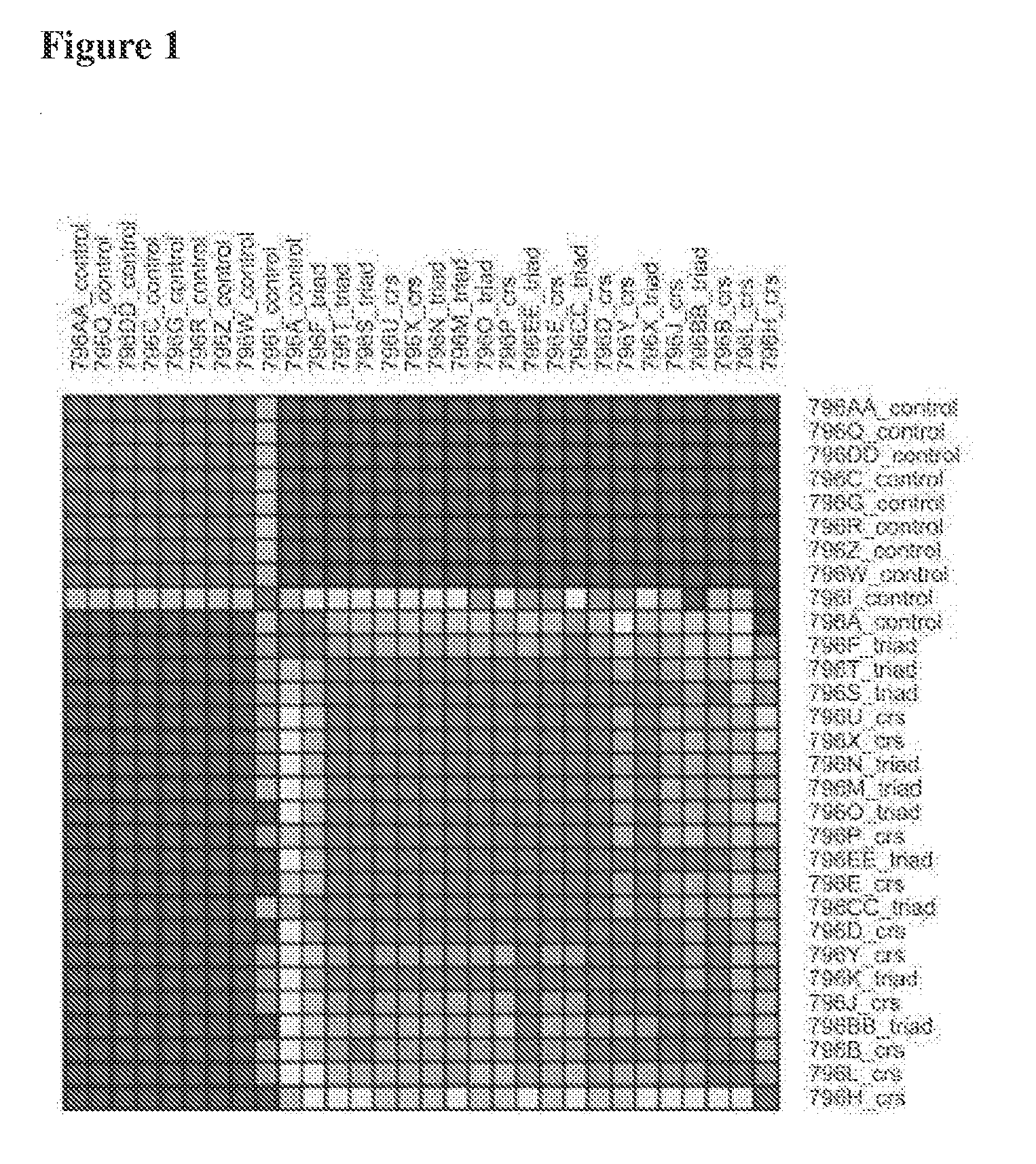
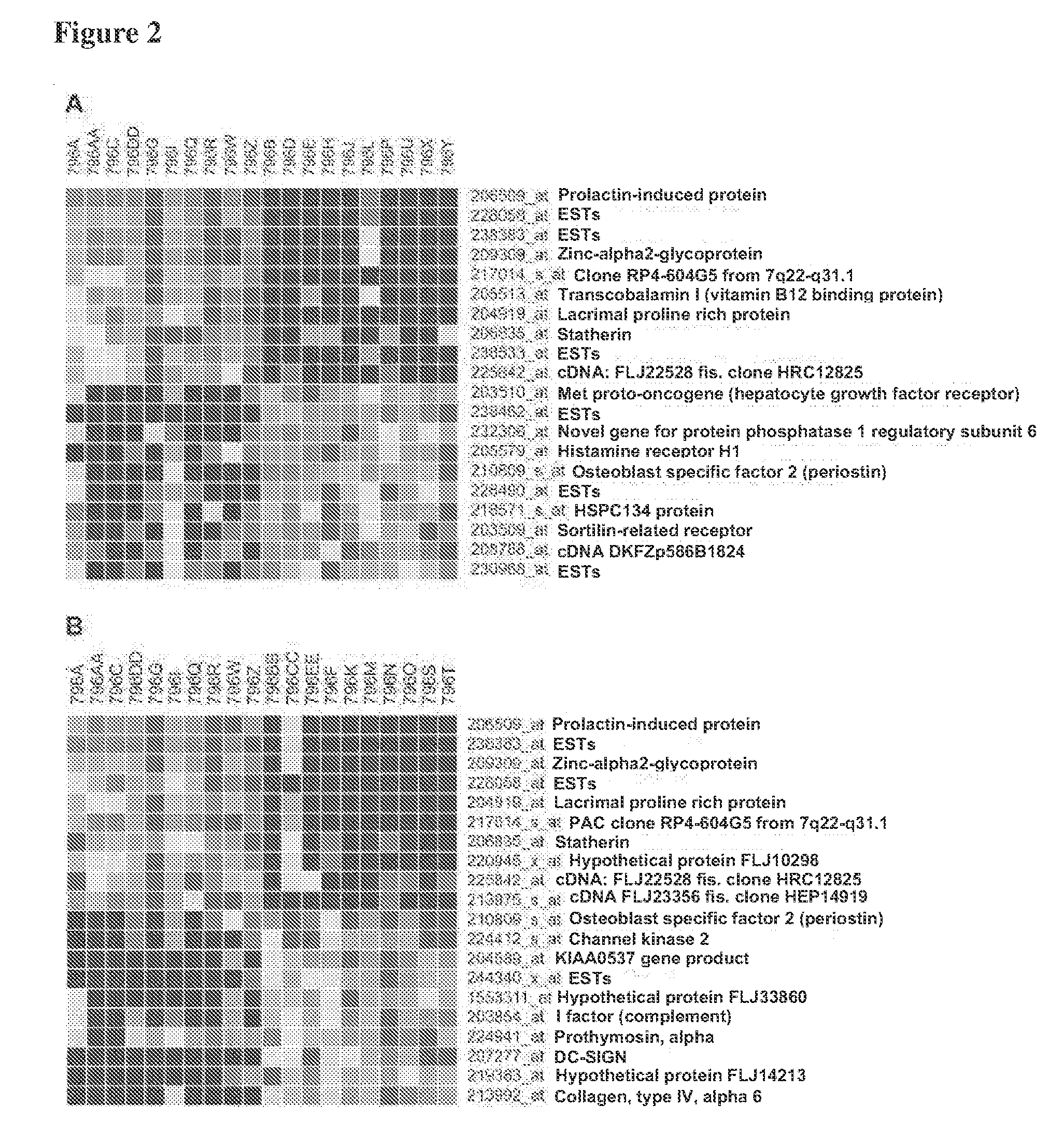
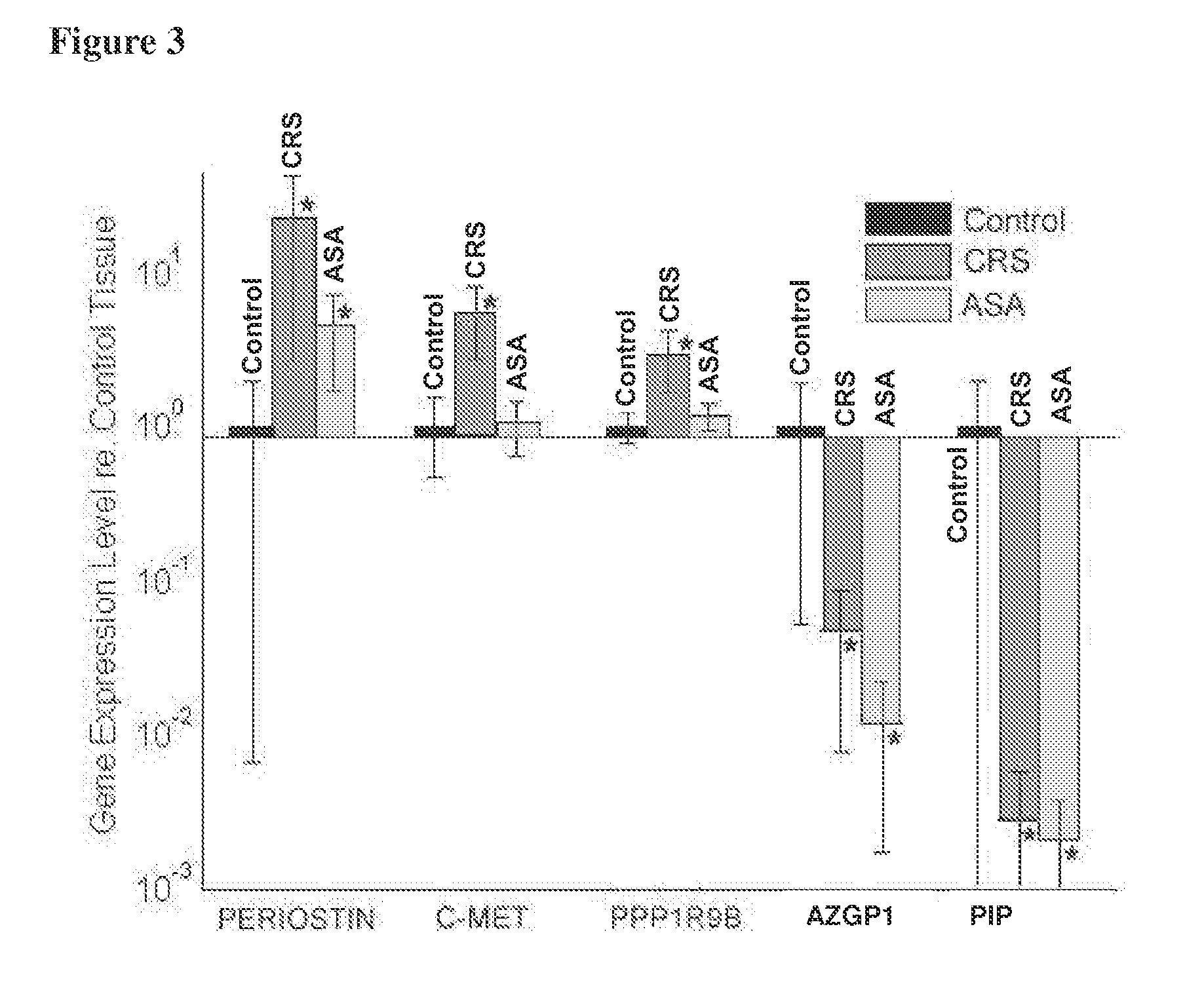
[0091]In this experiment, characteristic transcriptional signatures of chronic rhinosinusitis and aspirin-sensitive asthma were identified through genome-wide transcriptional profiling of nasal polyp tissue. Thirty genome-wide expression microarrays were used to compare nasal polyp tissue from patients with chronic rhinosinusitis alone (n=10) or chronic rhinosinusitis and a history of aspirin-sensitive asthma (n=10) to normal sinonasal mucosa from patients having non-sinus related conditions (n=10). Genes found to be most characteristic of each polyp phenotype, as determined from bioinformatic analyses, were then validated using real-time quantitative PCR and immunohistochemistry in a different set of patients. The experimental data show that transcriptional signature of the control mucosa was distinctly different from that of either polyp phenotype. Genes most characteri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| refractory | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com