Treatment of diseases and conditions mediated by increased phosphorylation
a technology of phosphorylation and disease, applied in the direction of antibacterial agents, peptide/protein ingredients, immunological disorders, etc., can solve the problems of inability to fully understand, so as to reduce the amount of atp and phosphate groups available, inhibit the effect of increased phosphorylation and increased phosphorylation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Inhibition of IL-8 By Dephosphorylated Phosvitin
[0216]Phosvitin was extracted from the yolks of chicken eggs as described by Losso and Nakai, in Egg Uses And Processing Technologies: New Developments, pages 150-157 (Sim and Nakai, eds., Cab International, Oxon, UK, 1994). It was dephosphorylated as described by Reimerdes and Klostermeyer, in Methods in Enzymology, Volume 45, pages 26-28 (1976). This phosvitin preparation was estimated to be about 20-50% dephosphorylated.
[0217]Chicken phosvitin was also purchased from Sigma Chemical Co., St. Louis, Mo., as was bovine serum albumin (BSA; BUS grade=low fatty acid and IgO). Each was used as received from the manufacturer without dephosphorylation.
[0218]Jurkat cells (American Type Culture Collection (ATCC), Rockville, Md.) were cultured (1×106 cells per 0.5 ml culture) in IMDM culture medium (ATCC) containing insulin transferrin selenite solution (ITSS; Sigma) with and without phosvitin or BSA at 37° C., 5% CO2, for 30 minutes. Then, pho...
example 2
Use of Dephosphorylated Phosvitin to Treat Inflammation
[0221]Chicken phosvitin (Sigma) in distilled water (0.5 g / 100 ml) was dephosphorylated by adding 0.4 N NaOH at 37° C. for 3 hours as described in Jiang et al., J. Agric. Food Chem., 48:990-994 (2000). After dephosphorylation, the pH of the phosvitin solution was adjusted to 7.8 with 0.1 N HCl. This phosvitin preparation was found to be 73% dephosphorylated using a gallium column.
[0222]A cream was prepared by mixing the dephosphorylated phosvitin with 100 ml Eucerin cream (Beiersdorf Inc., Wilton, Conn.) to give a final phosvitin concentration of about 0.25%.
[0223]A human volunteer with a chronic skin lesion (etiology unknown) applied the phosvitin cream to the lesion twice per day for two days. The lesion disappeared completely after this treatment.
[0224]Another human volunteer with chronic skin lesions (diagnosed as atopic dermatitis) applied the phosvitin cream to a lesion on the back twice per day for one week. The treated le...
example 3
Inhibition of IL-8 by Unphosphorylated Kinase Substrates
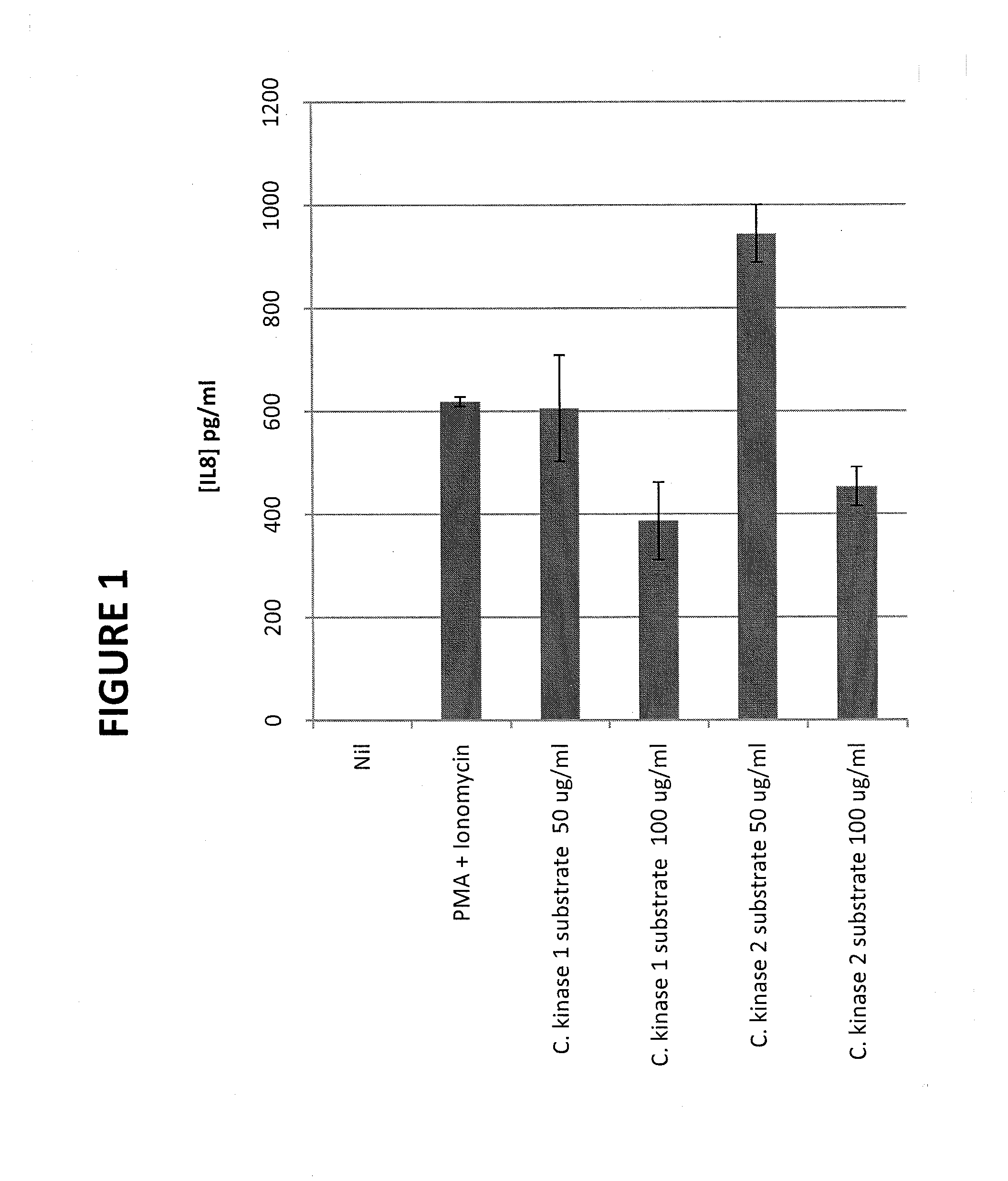
[0228]Casein kinase I substrate (sequence Arg Arg Lys Asp Leu H is Asp Asp Glu Glu Asp Glu Ala Met Ser Ile Thr Ala [SEQ ID NO:3]) and casein kinase II substrate (sequence Arg Arg Arg Ala Asp Asp Ser Asp [SEQ ID NO:4]) were purchased from Sigma-Aldrich, St. Louis, Mo. Each was used as received from the manufacturer since they were unphosphorylated.
[0229]Jurkat cells (American Type Culture Collection (ATCC), Rockville, Md.) were cultured (2×106 cells per 1.0 ml culture) in IMDM culture medium (ATCC) containing insulin transferrin selenite solution (ITSS; Sigma) with and without each of the casein kinase substrates (50 μg / ml and 100 μg / ml) at 37° C., 10% CO2, for 15 minutes. Then, phorbol-12-myristate-13-acetate (PMA; 100 mg / ml; Sigma) and ionomycin (100 mg / ml; Sigma) were added to stimulate the cells, and the cells were further cultured at 37° C., 5% CO2, for 24 hours.
[0230]Cell culture supernatants were analyzed for IL-8 content...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com