Lyme disease vaccine
a technology for lyme disease and vaccine, applied in the field of lyme disease vaccine and diagnostic, can solve the problems of ineffective use of full-length ospa, high socio-economic cost of lyme disease, and inability to provide an efficacious vaccine for use in the prevention and/or treatment of lyme disease, etc., and achieve the effect of broad protection against infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
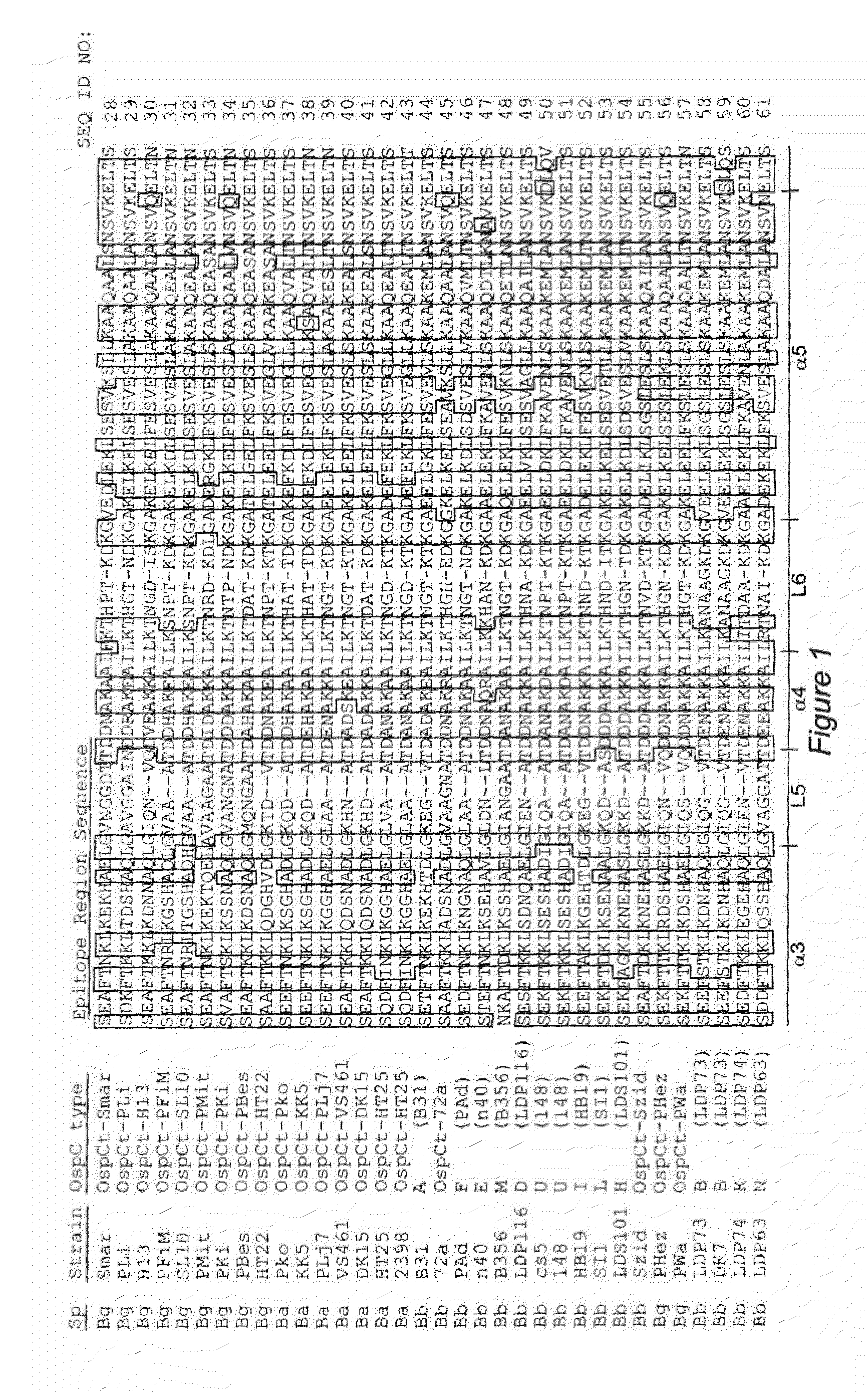
Identification of Linear Immunodominant Epitopes of Borrelia OspA Protein
Materials and Methods
[0073]Recombinant protein production
[0074]Fragments of the outer surface protein A (OspA) gene were amplified by PCR using high fidelity DNA polymerase (Phusion HF, New England Biolabs), and the amplicons purified by agarose gel electrophoresis and gel extraction. Overhangs were generated by treatment with T4 polymerase to allow ligase-independent annealing to the pET-32 Ek / LIC vector. The annealed vector was transformed into Escherichia coli, and the transformants were selected for ampicillin resistance, and were screened for the presence of the OspA gene fragment by PCR. Plasmids were then extracted from the transformants and the sequence confirmed by DNA sequencing (MWG Biotech). The plasmids were then used to transform E. coli BL21 (DE3) cells for protein production.
[0075]To generate recombinant proteins, BL21(DE3) cells were grown at 37° C. to an OD600 of 0.5, then 1 mM IPTG was added ...
example 2
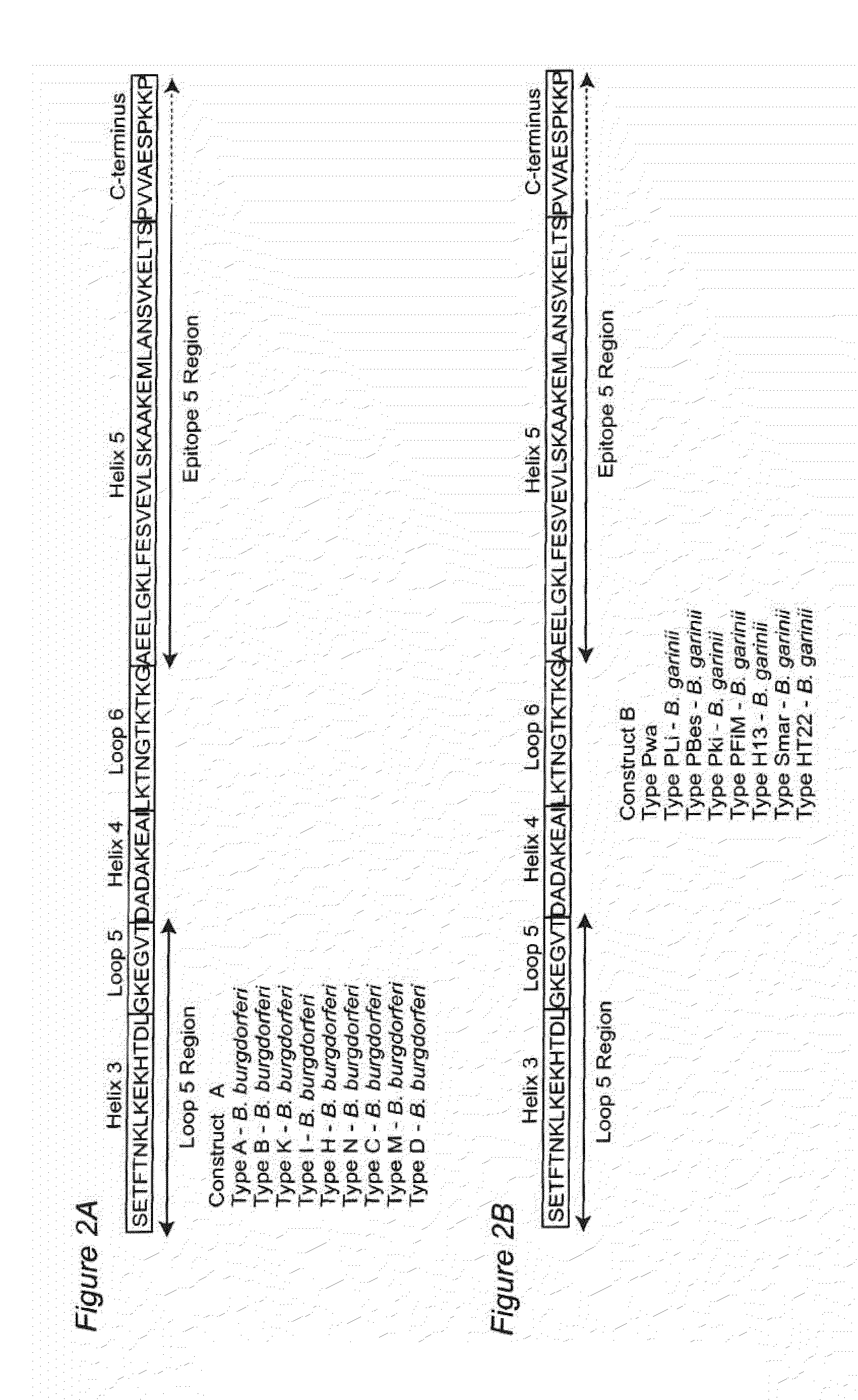
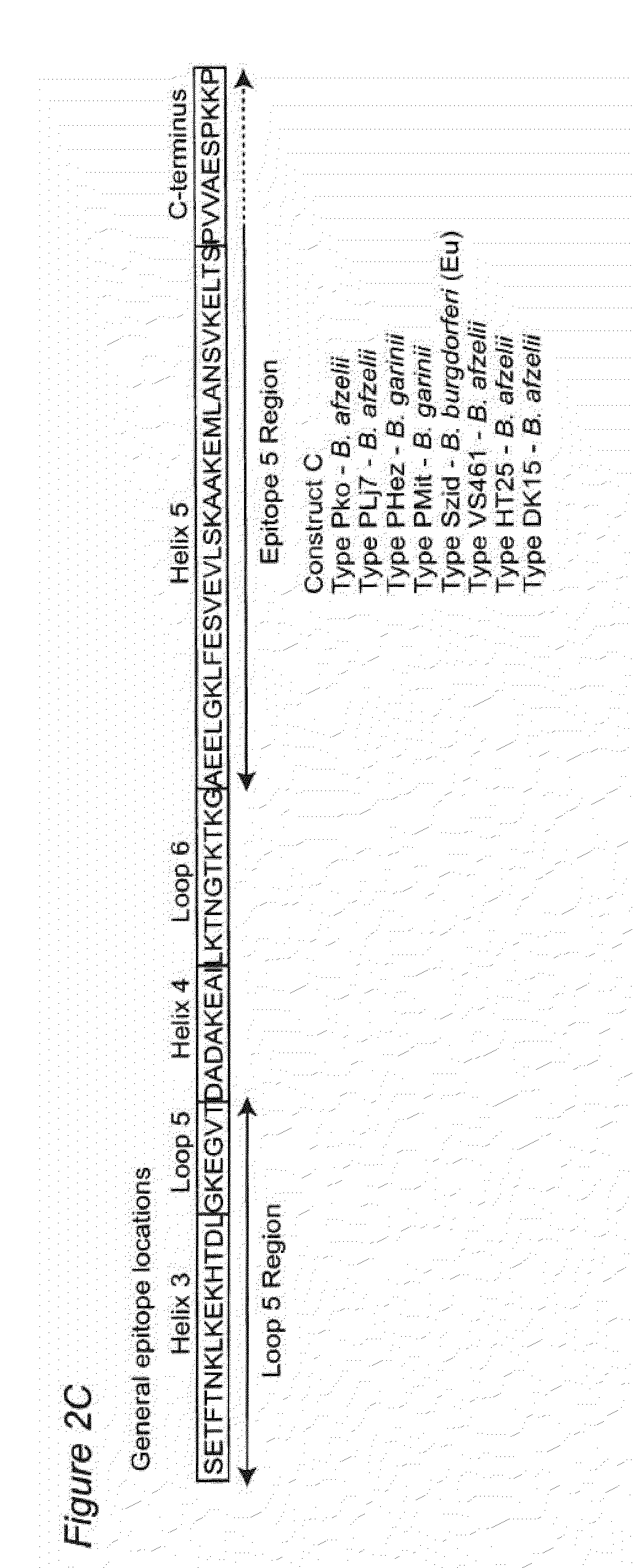
[0083]When administered to test mammals, this chimeric polypeptide construct comprising at least one 221-240 epitope containing region, and usually two or more 221-240 epitope containing regions from of different phyletic types, is found to elicit a robust immune response, and to provide protection from the development of Lyme disease.
[0084]While the invention has been described in terms of its preferred embodiments, those skilled in the art will recognize that the invention can be practiced with modification within the spirit and scope of the appended claims. Accordingly, the present invention should not be limited to the embodiments as described above, but should further include all modifications and equivalents thereof within the spirit and scope of the description provided herein.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Antigenicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com