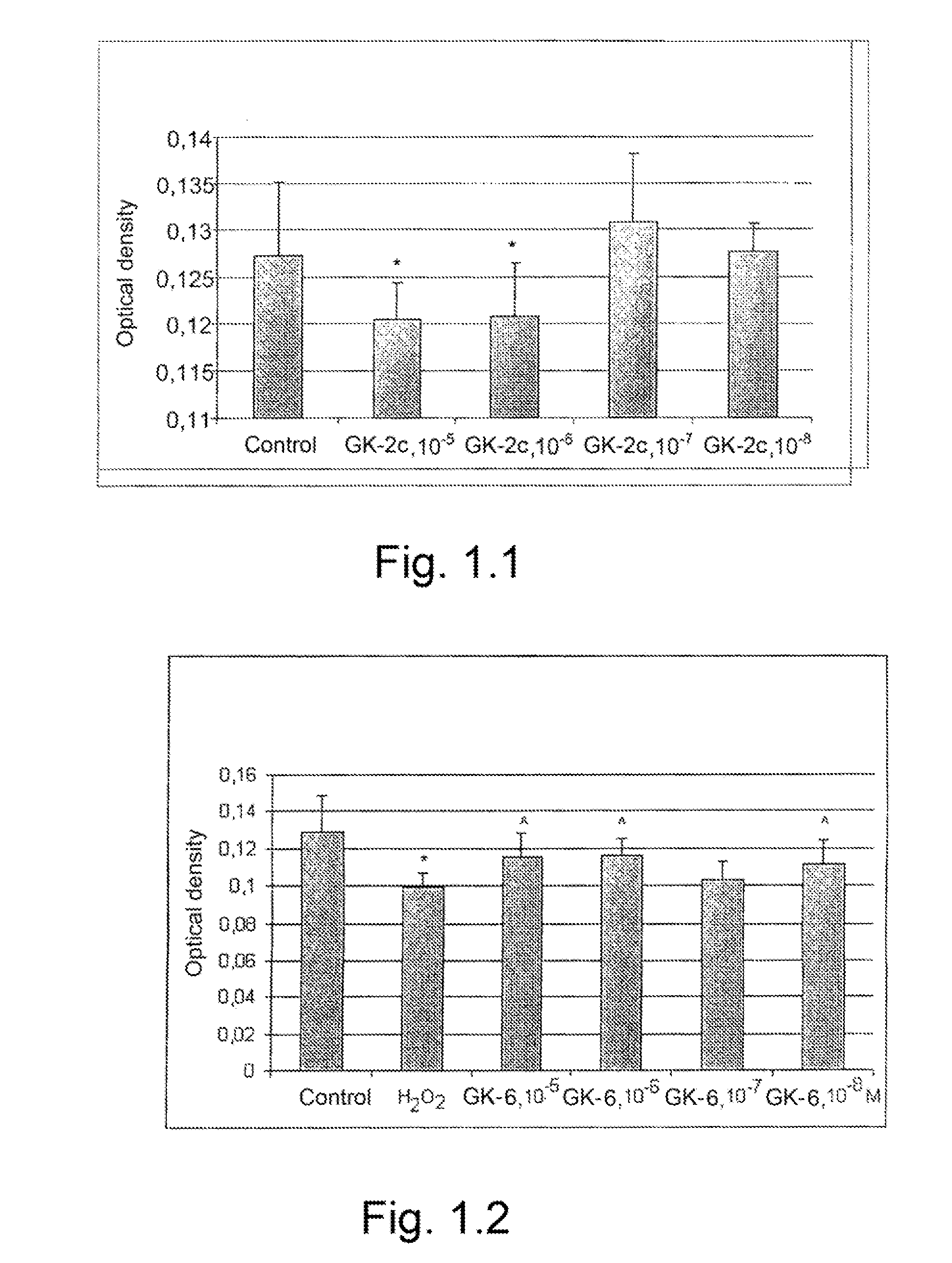
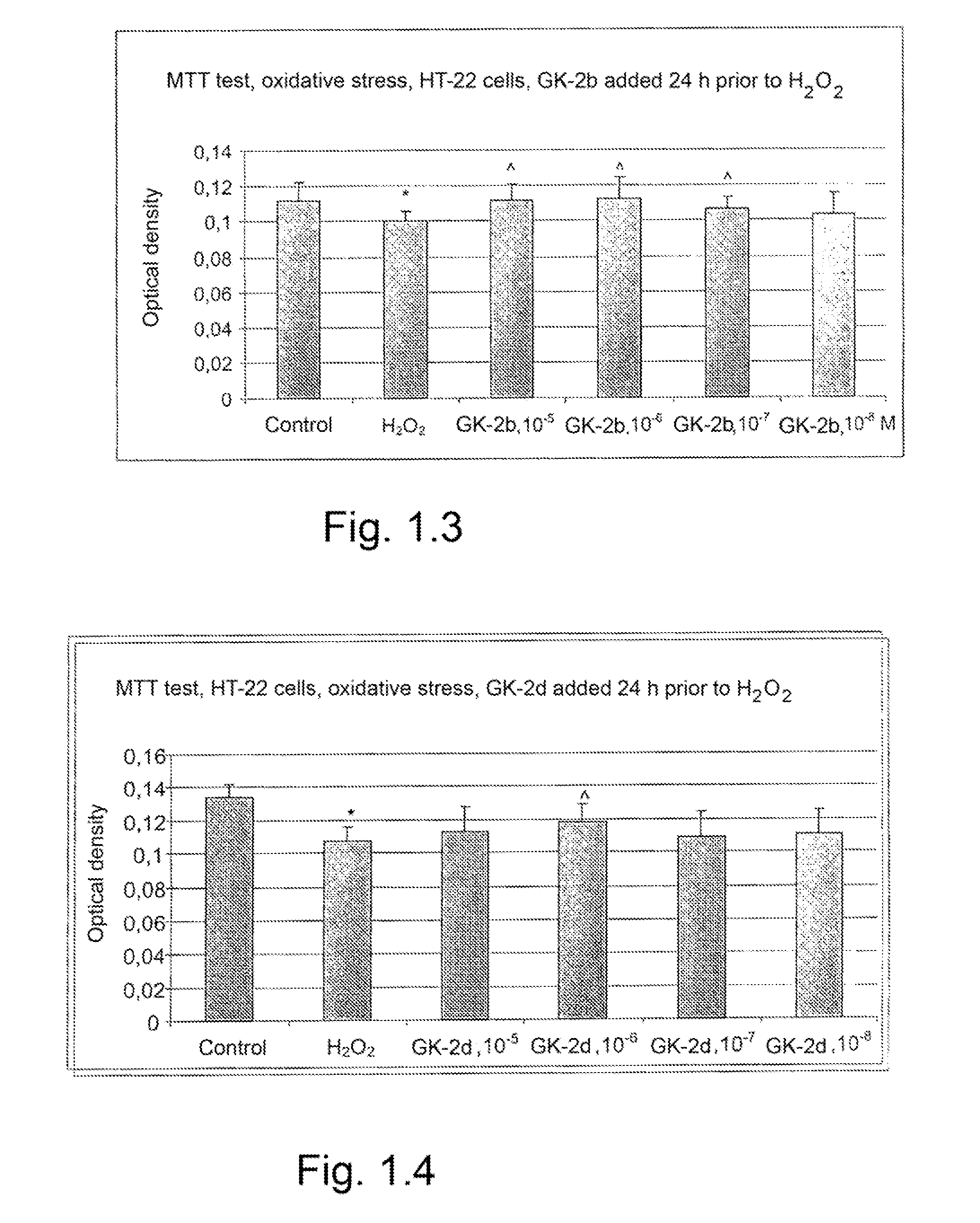
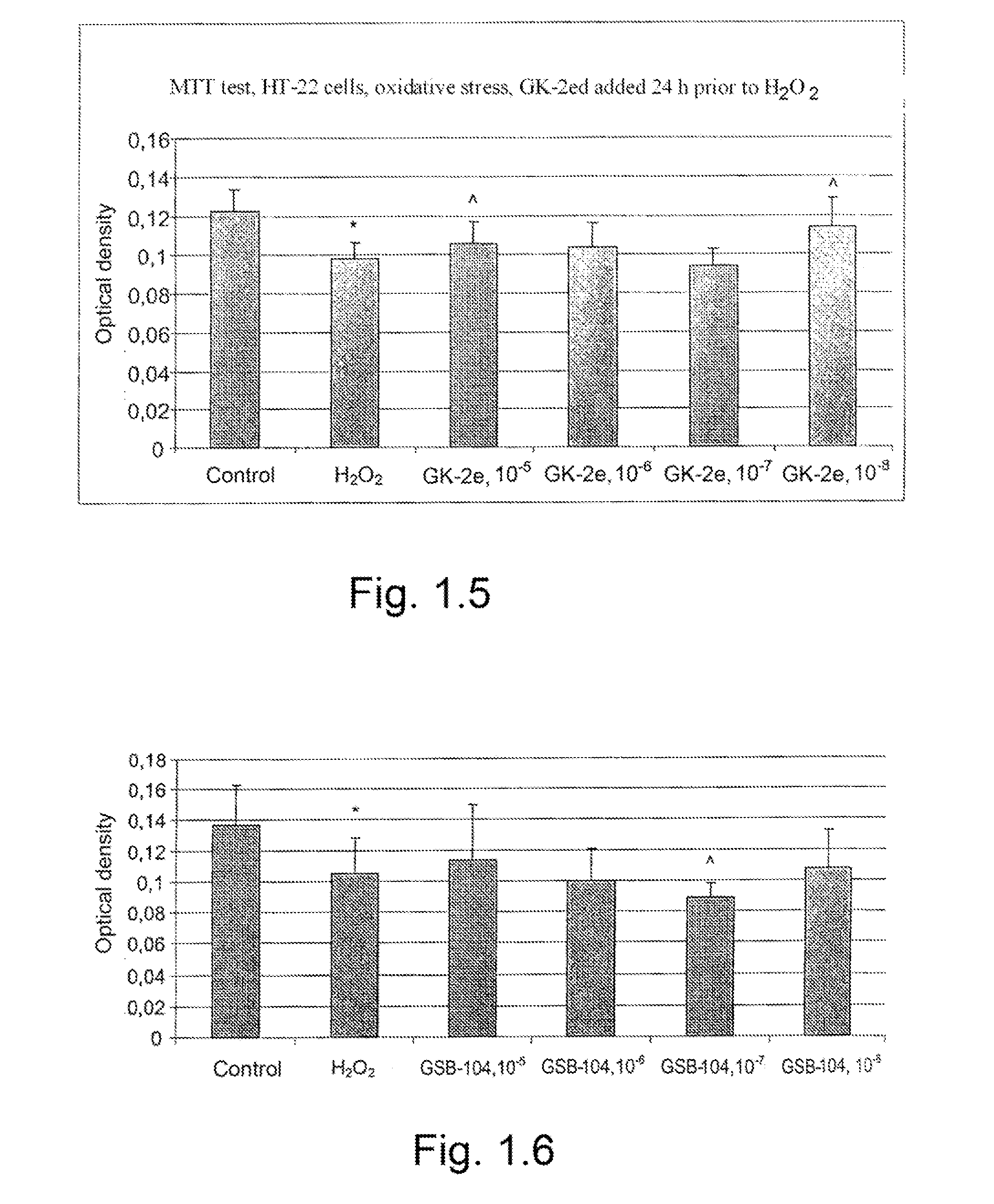
Dipeptide mimetics of ngf and bdnf neurotrophins
a neurotrophin and dipeptide technology, applied in the field of bioorganic chemistry, can solve the problems of rapid degradation of neurotrophins in the bloodstream, development of pain syndrome upon their use, and failure of neurotrophin drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
The Synthesis of N-monosuccinyl-glutamyl-lysine amide, HOOC(CH2)2CO-Glu-Lys-NH2 (GK-1)
a) The Preparation of N-benzyloxycarbonyl-γ-t-butyl-glutamyl-Nε-t-butyloxycarbonyl-lysine amide, Z-Glu(OBut)-Lys(Boc)-NH2
[0137]A solution of 4.0 g (8.4 mmol) N-benzyloxycarbonyl-Nε-tert-butyloxycarbonyl-lysine N-oxysuccinimide ester (Z-Lys(Boc)-OSu) in 20 ml DMFA was treated with 2 ml conc. ammonia for 5 min followed by dilution with water, and the precipitated N-benzyloxycarbonyl-Nε-tert-butyloxycarbonyl-lysine amide (Z-Lys(Boc)-NH2) was filtered off. The resulting product was dissolved in methanol, then 3.0 g of 10% Pd / C was added and hydrogenated at room temperature. The catalyst was filtered off, the solvent was removed in vacuo, and the residue was taken up in 20 ml DMFA. To this solution 3.9 g (9.0 mmol) N-benzyloxycarbonyl-γ-t-butyl-glutamic acid N-oxysuccinimide ester (Z-Glu(OBut)-OSu) was added. The reaction mixture was stirred for 6 h at room temperature (TLC control), then 2 ml DMPDA w...
example 2
The Synthesis of bis-(N-monosuccinyl-glutamyl-lysine)hexamethylenediamide, (HOOC(CH2)2CO-Glu-Lys-NH)2—(CH2)6 (GK-2)
a) The Preparation of bis-(N-benzyloxycarbonyl-Nε-t-butyloxycarbonyl-lysine)hexamethylenediamide, (Z-Lys(Boc)-NH)2—(CH2)6
[0141]A solution of 4.3 g (9.0 mmol) N-benzyloxycarbonyl-Nε-t-butyloxycarbonyl-lysine N-oxysuccinimide ester (Z-Lys(Boc)-OSu) and 0.5 g (4.3 mmol) hexamethylenediamine in 20 ml DMFA was stirred for 4 h at room temperature, forming a cloudy solution. The reaction mixture was diluted with 80 ml water and allowed to stand for a few hours. The solidified precipitate was filtered off and washed with water. It was used without drying.
b) The Preparation of bis-(N-benzyloxycarbonyl-γ-t-butyl-glutamyl-Nε-t-butyloxycarbonyl-lysine)hexamethylenediamide, (Z-Glu(OBut)-Lys(Boc)-NH)2—(CH2)6
[0142]The (Z-Lys(Boc)-NH)2—(CH2)6 prepared in (a) was hydrogenated in methanol over 10% Pd / C at room temperature. When the starting compound disappeared (TLC control), the ca...
example 3
The Synthesis of N-acetyl-lysyl-glutamic acid amide, CH3CO-Lys-Glu-NH2 (GK-3)
a) The Preparation of γ-benzyl-glutamic acid amide, H-Glu(OBzl)-NH2
[0146]8.0 g (17.4 mmol) of N-t-butyloxycarbonyl-γ-benzyl-glutamic acid p-nitrophenyl ester (Boc-Glu(OBzl)-ONp) were dissolved in 30 ml DMFA, 3.5 ml aqueous ammonia was added and allowed to stand for 5 min. Then to the reaction mixture 100 ml water, 50 ml diethyl ether and 100 ml of hexane were added and allowed to stand at +5° C. for a few hours. The precipitate was filtered off, and the solid residue was washed with water and hexane. The resulting product was dissolved in 80 ml TFA, and the solution was kept for 1 h at room temperature. The solvent was removed in vacuo, and the residue was allowed to crystallize with ether. The residue that crystallized gradually was filtered off and washed with ether to yield 5.8 g (96%) of the product. M.p. 68-70° C., [α]6D+10.0° (c=0.3; water-ethanol, 1:2).
b) The Preparation of N-t-butyloxycarbonyl-Nε-b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com