Salts or Co-Crystals of 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol
a technology of dimethylamino-1 ethyl-2-methylpropyl and cocrystal, which is applied in the field of salt or cocrystal of 3(3dimethylamino1ethyl2methylpropyl)phenol, can solve the problems of undesirable variations, rapid onset of analgesic action, and detrimental to patient complian
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example b1-1
Salt or Cocrystal of Tapentadol and (2S,3S)-Dibenzoyl Tartaric Acid
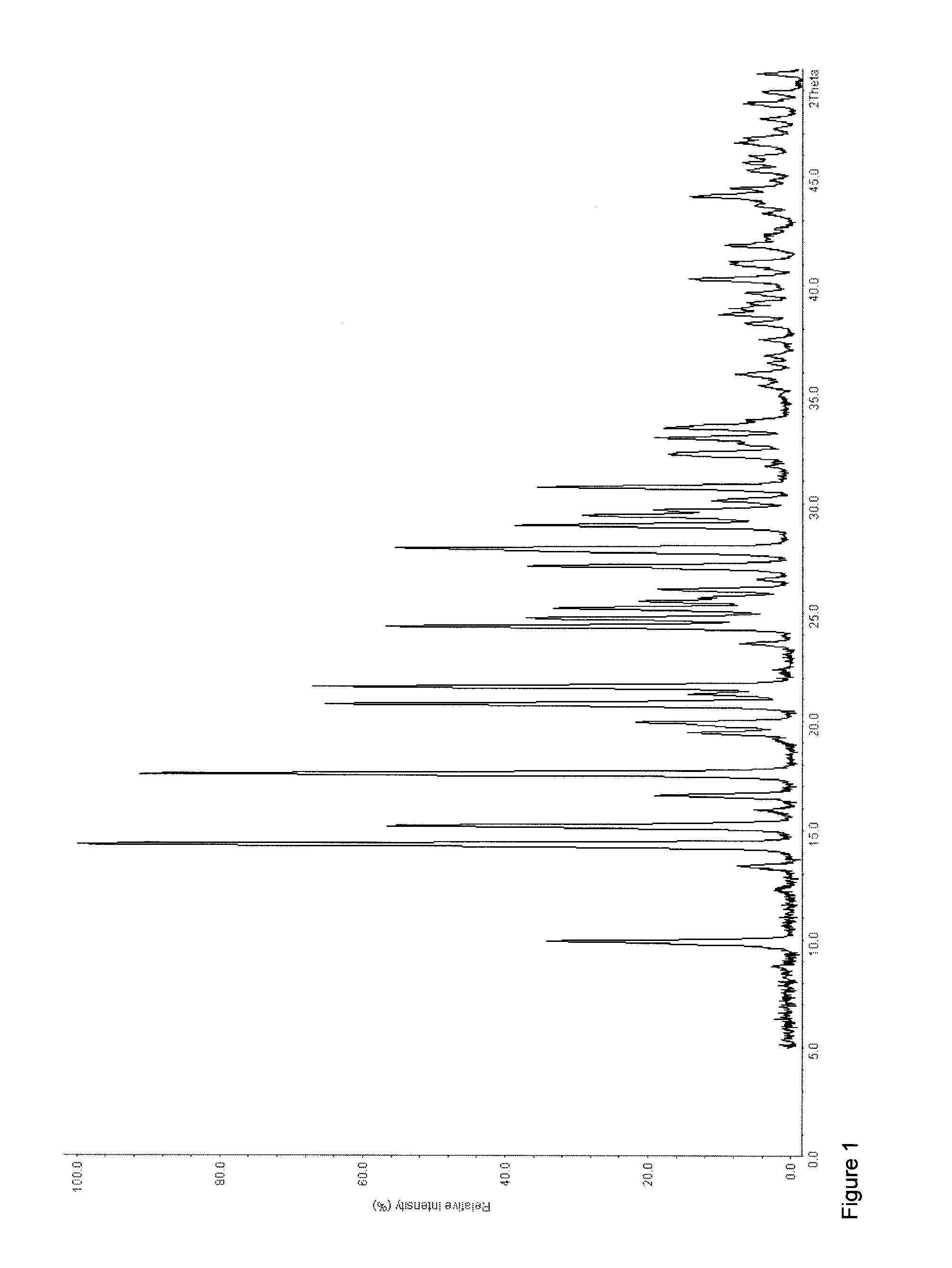
[0220]20 g (0.090 mol) of tapentadol and 16.19 g (0.045 mol) of (+)-(2S,3S) dibenzoyl tartaric acid were dissolved in 400 mL of acetone. The mixture was stirred at room temperature for about 3 hours. The resulting crystalline precipitate was filtered off and dried under reduced pressure (6 mbar) at 40° C. (yield 36.1 g, 100%, melting point (DSC): To=152.5° C., Tp=170.7° C.; 9.7 J / g, To=185.7° C., Tp=188.2° C., 119.1 J / g). 1H-NMR analysis showed a 2:1-stochiometry of tapentadol and (+)-(2S,3S) dibenzoyl tartaric acid.
example b1-2
Salt or Cocrystal of Tapentadol and Sebacic Acid
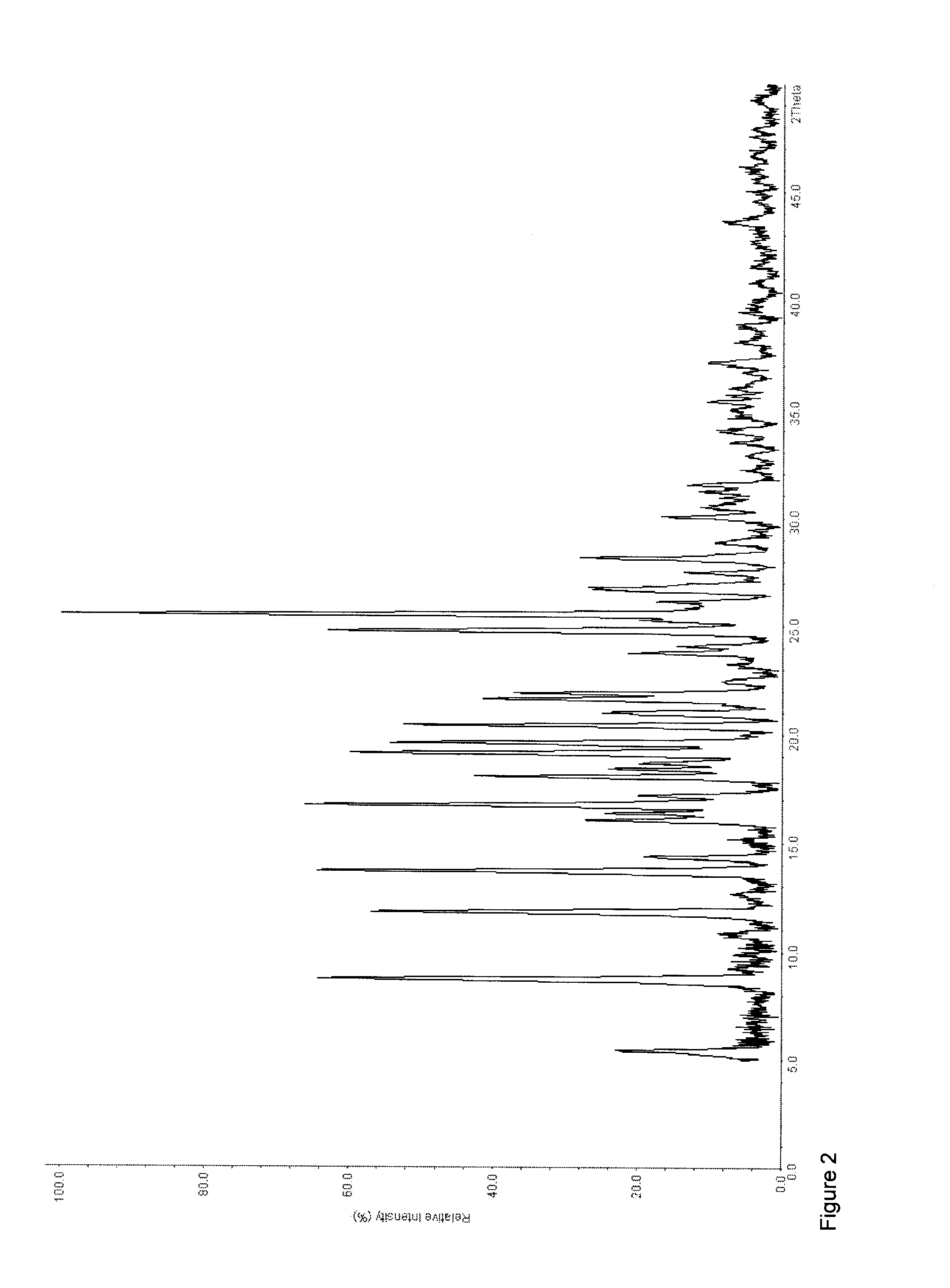
[0221]125 g (0.56 mol) of tapentadol were dissolved in 700 mL of ethyl acetate. To this solution, 114.21 g (0.56 mol) of sebacic acid were added as a solid in portions. Then, 50 mL of ethyl acetate were added. Subsequently, the resulting suspension was stirred for 15 hours. The resulting crystalline white precipitate was then slowly filtered off and dried at 60° C. under reduced pressure (6 mbar) (yield: 232.96 g, 97.37%, melting point (DSC): To=77.9° C., Tp=81.1° C., 99.5 J / g). 1H-NMR analysis showed a 1:1-stochiometry of tapentadol and sebacic acid.
example b1-3
Salt or Cocrystal of Tapentadol and 1-Hydroxy-2-Naphthoic Acid
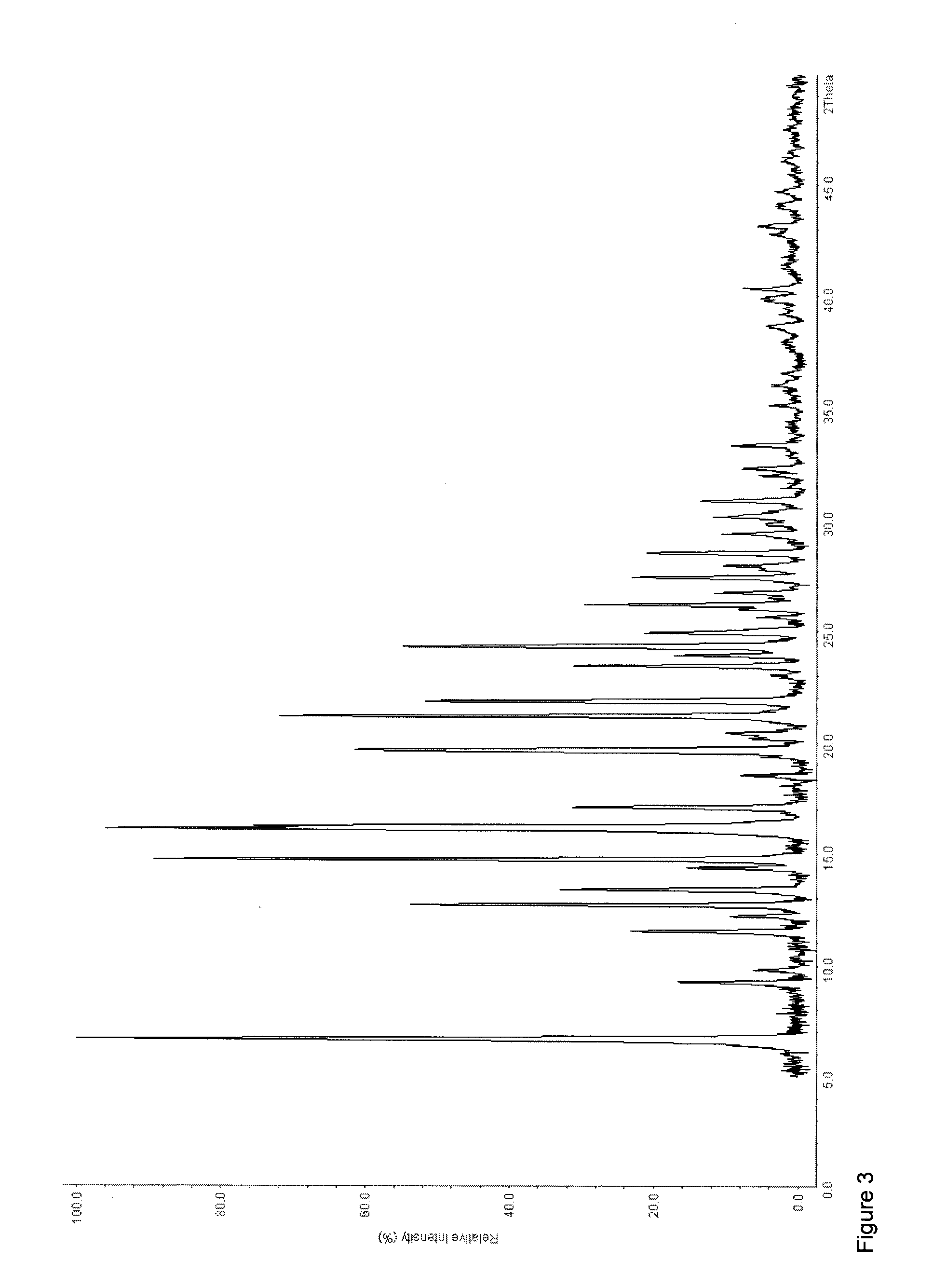
[0222]120 g (0.54 mol) of tapentadol were dissolved in 700 mL of 2-propanol. To this solution, 102.02 g (0.54 mol) of 1-hydroxy-2-naphthoic acid were added as a solid in portions. Then, 100 mL of 2-propanol were added. After complete addition, the resulting suspension was stirred for 18 hours. The crystallized brownish precipitate was then filtered off and dried at 50° C. under reduced pressure (6 mbar) (yield: 199.43 g, 89.83%, melting point (DSC): To=114.9° C., Tp=122.7, ° C., 1.0 J / g; To=157.6° C., Tp=159.9° C., 78.3 J / g). 1H-NMR analysis showed a 1:1-stochiometry of tapentadol and 1-hydroxy-2-naphthoic acid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com