Methods For Detection Of Gastric Cancer
a gastric cancer and gastric cancer technology, applied in the field of gastric cancer detection methods, can solve the problems of its cost effectiveness remaining an issue, and the discovery of biomarkers in body fluids is not without challenges, so as to improve the outcome of gastric cancer patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
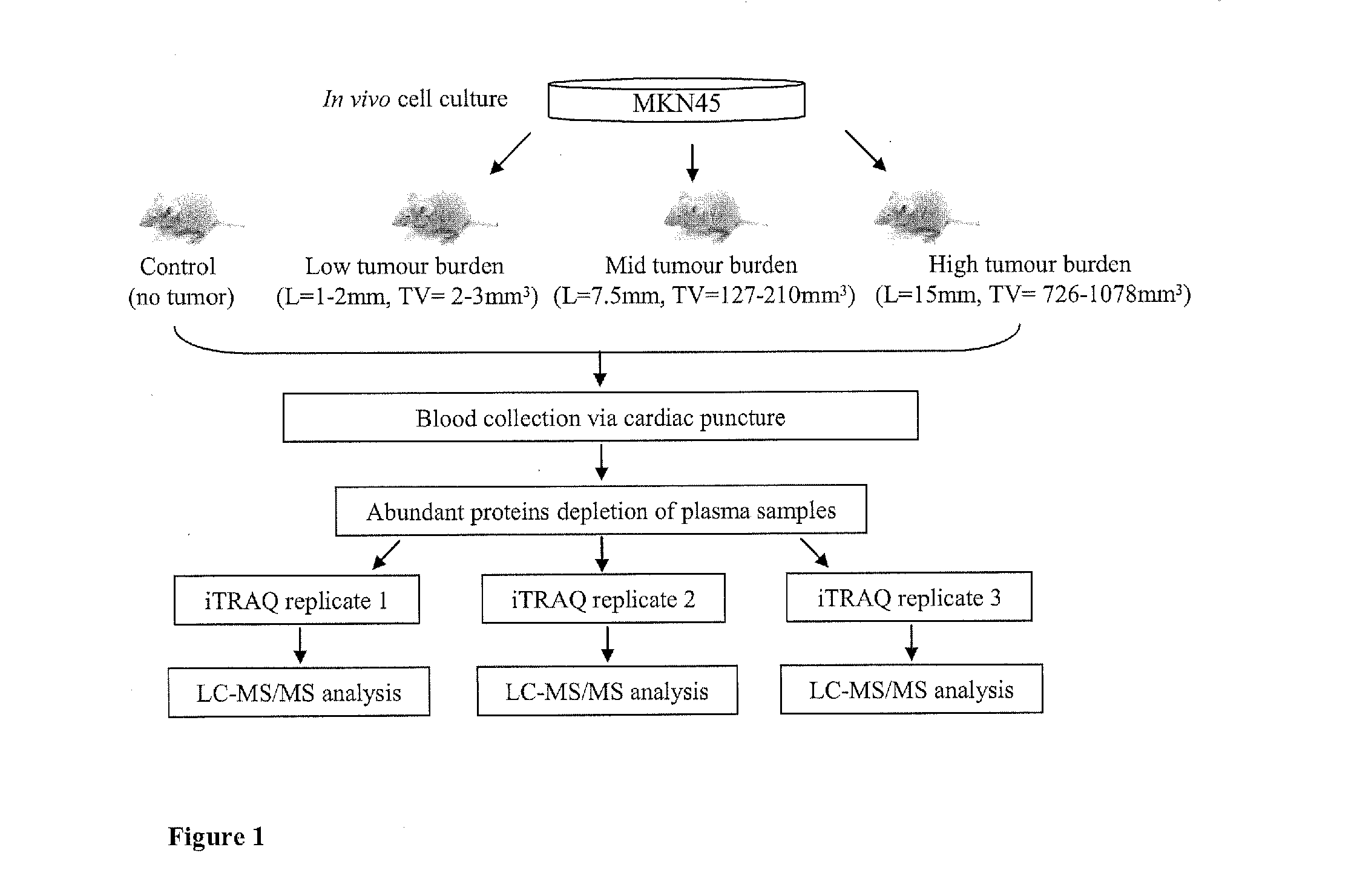
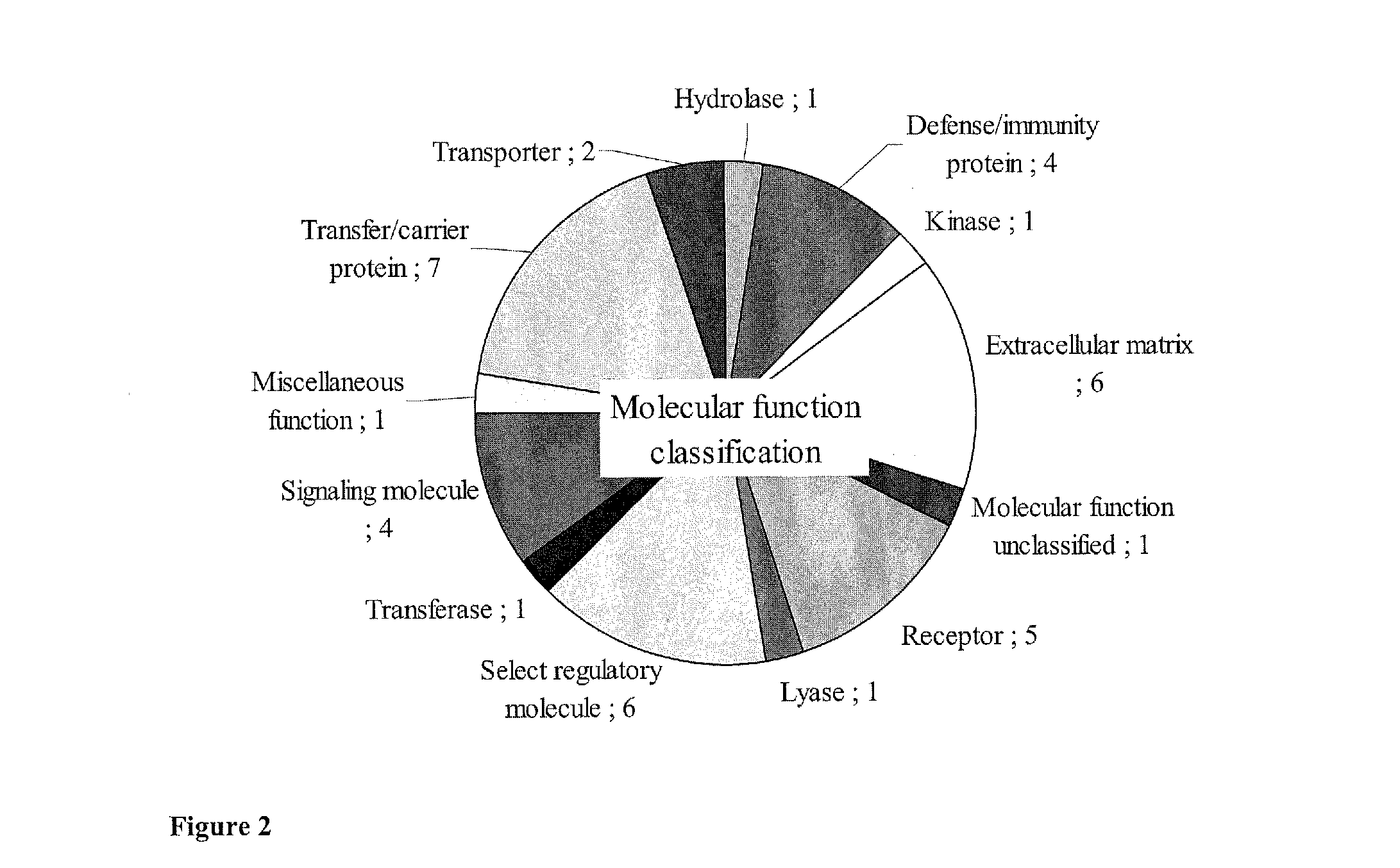
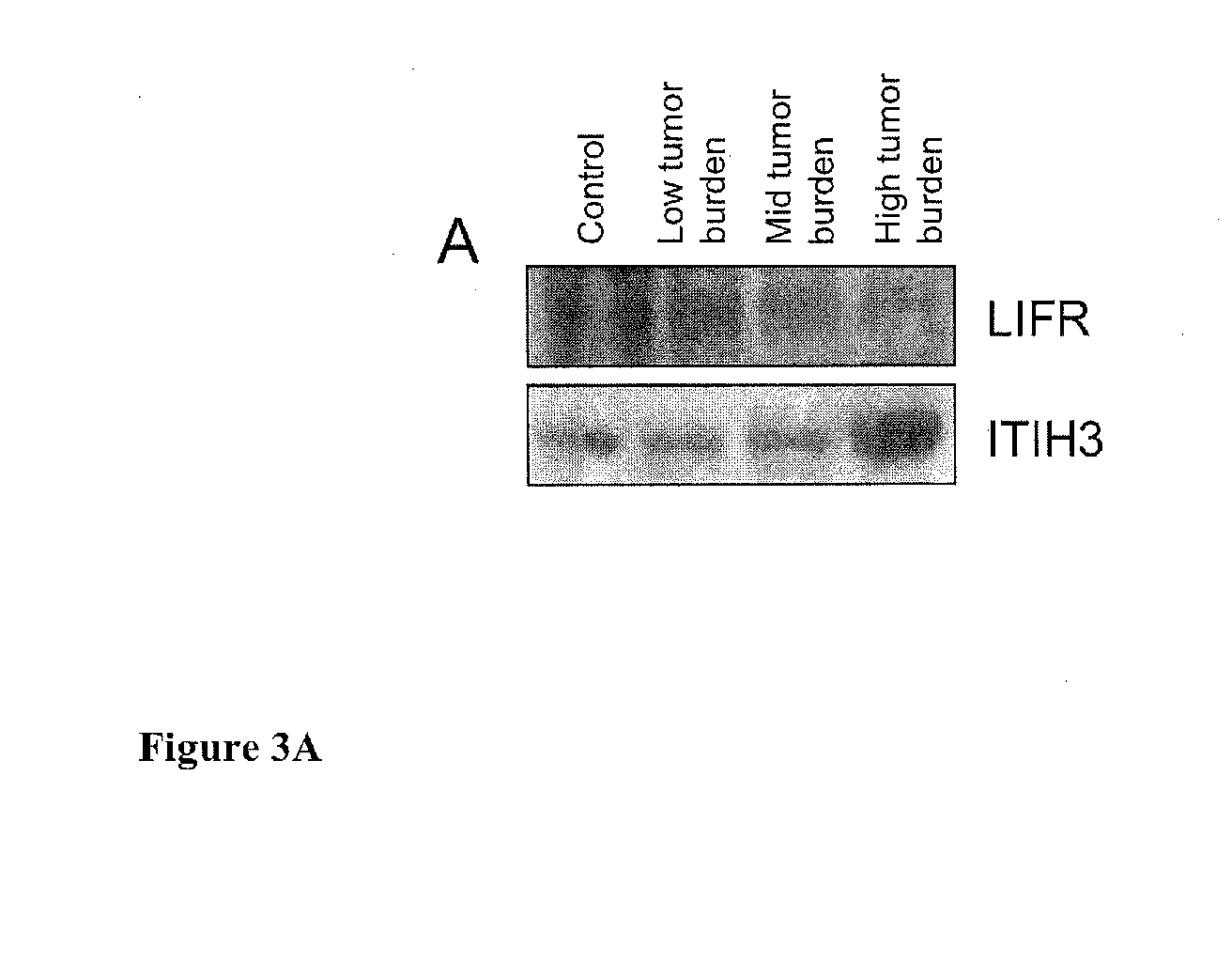
[0025]A mouse xenograft model is an alternative to analysis of clinical samples for biomarker discovery. It presents less genetic and biological variations compared to a human model at the same time allowing environmental factor to be tightly controlled (Frese, K K.; Tuveson, D. A., Nat Rev Cancer 2007, 7, (9), 645-58). Another advantage is that the xenograft model tends to have bigger tumor / blood ratio than humans, thereby enabling easier detection of cancer-induced changes. The feasibility of using a xenograft model for biomarker discovery had been demonstrated by various serum biomarker studies including that for breast (Pitteri, S. et al., J Proteome Res 2008, 7, (4), 1481-9), pancreas (Faca, V. M. et al., PLoS Med 2008, 5, (6), e123), stomach (Juan, H. F. et al., Proteomics 2004, 4, (9), 2766-75) and prostate (van Weerden, W. M.; Romijn, J. C., Prostate 2000, 43, (4), 263-71). For example, identification of nm23 / nucleoside-diphosphate kinase and human glycolytic enzyme in prost...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com