Heparan sulfate synthesis
a technology of heparan sulfate and sulfate, which is applied in the direction of sugar derivates, organic chemistry, chemistry apparatus and processes, etc., can solve the problems of hs-binding motifs that cannot be identified, unable to determine the structure of binding epitopes, and hs-binding motifs are difficult to identify, etc., to achieve reliably and economically produced effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
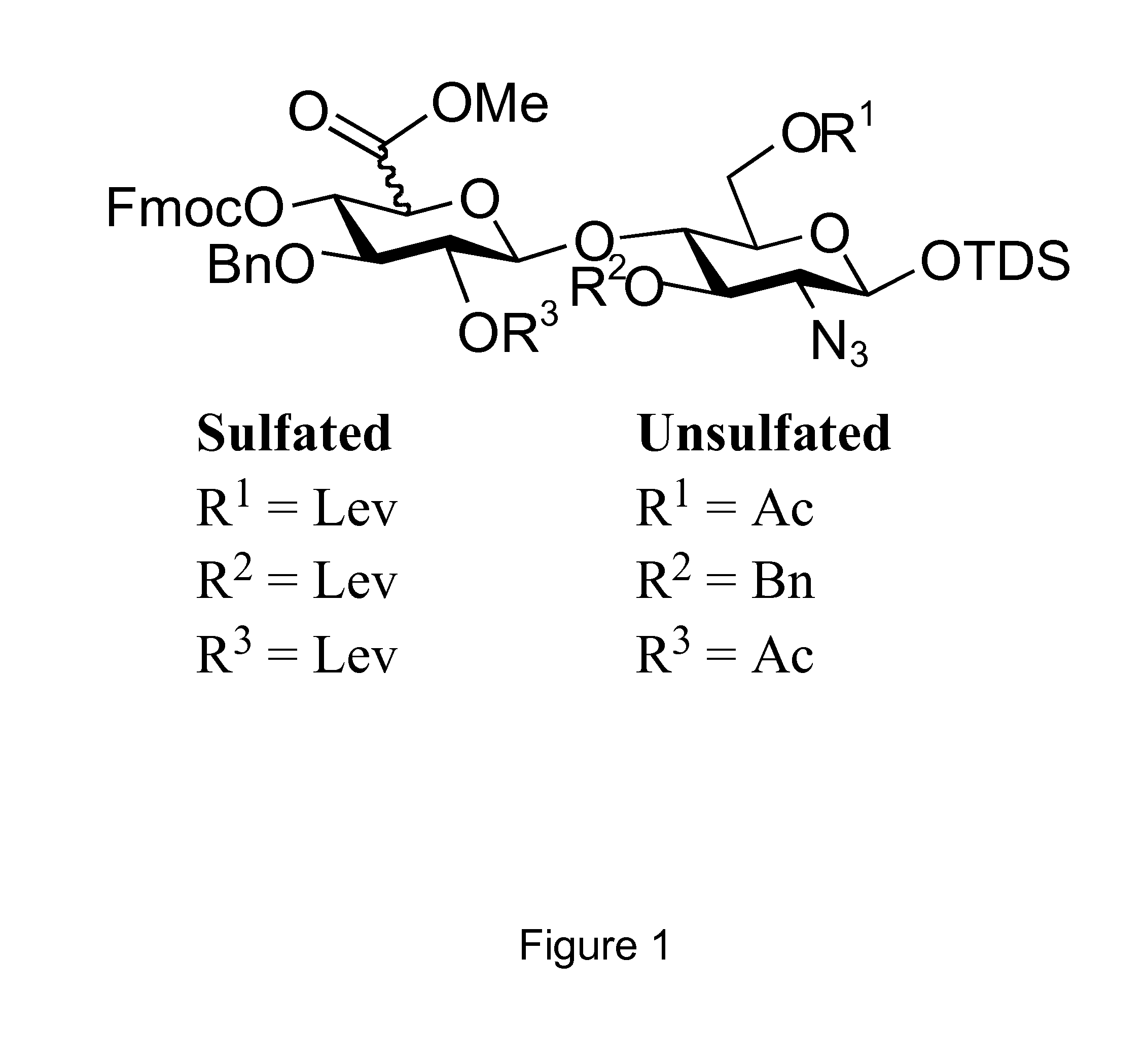
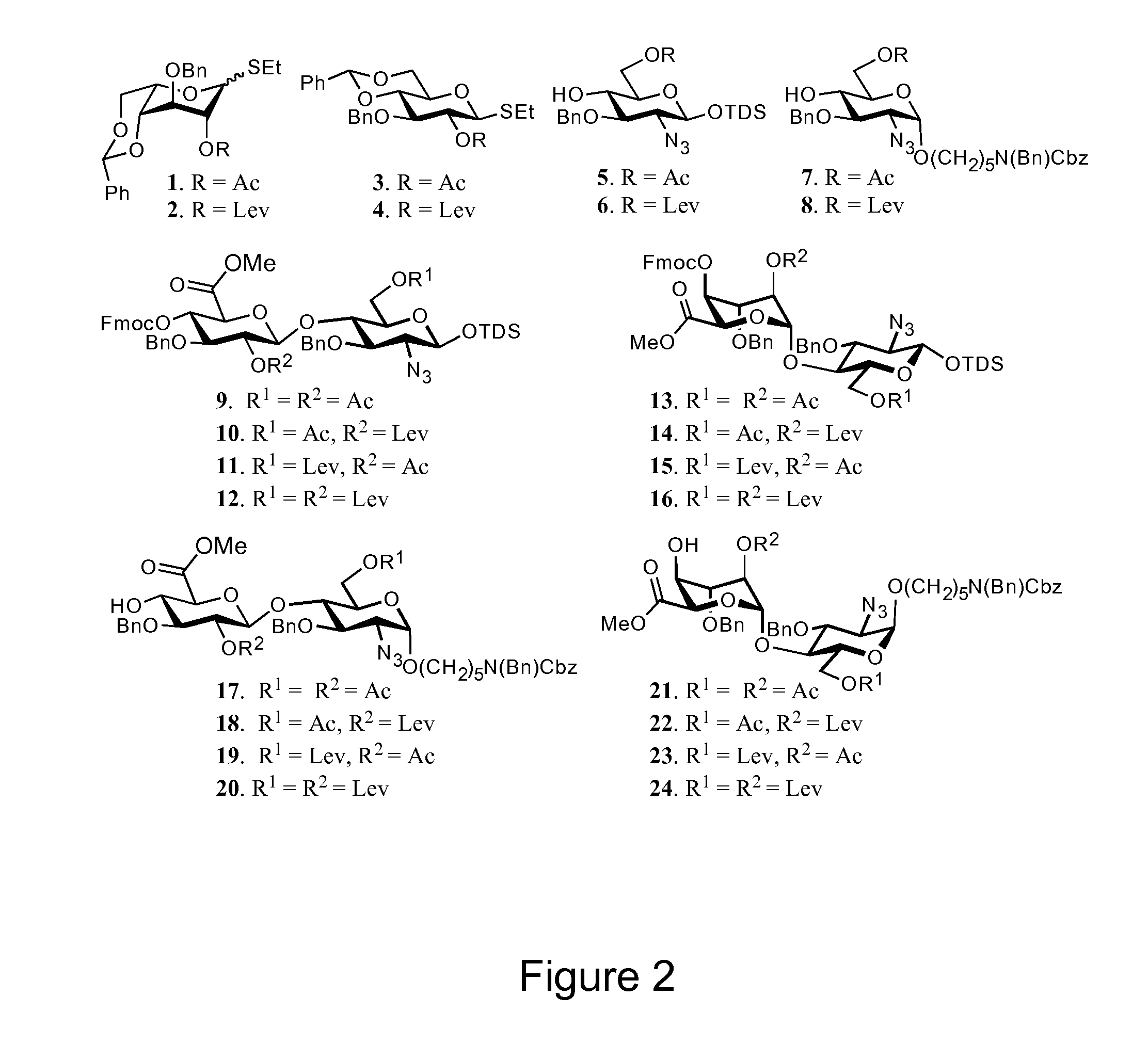
Modular Synthesis of Heparan Sulfate Oligosaccharides for Structure-Activity Relationship Studies
[0128]Although hundreds of heparan sulfate binding proteins have been identified, and implicated in a myriad of physiological and pathological processes, very little information is known about ligand requirements for binding and mediating biological activities by these proteins. This difficulty results from a lack of technology for establishing structure-activity-relationships, which in turn is due to the structural complexity of natural heparan sulfate (HS) and difficulties of preparing well-defined HS-oligosaccharides. To address this deficiency, we have developed a modular approach for the parallel combinatorial synthesis of HS oligosaccharides that utilizes a relatively small number of selectively protected disaccharide building blocks, which can easily be converted into glycosyl donors and acceptors. The utility of the modular building blocks has been demonstrated by the preparation...
example ii
Experimental Procedure for Synthesis and Characterization of Heparan Sulfate Oligosaccharides
General Procedures
[0190]All moisture sensitive reactions were performed under an argon atmosphere by using vacuum dried glassware. All commercial materials were used without purification, unless otherwise noted. CH2Cl2 was freshly distilled from calcium hydride under nitrogen prior to use. Toluene, DMF, diethylether, methanol and THF were purchased anhydrous and used without further purification. Molecular sieves (4 Å) were flame activated in vacuo prior to use. All reactions were performed at room temperature unless specified otherwise. TLC-analysis was conducted on Silica gel 60 F254 (EMD Chemicals Inc.) with detection by UV-absorption (254 nm) were applicable, and by spraying with 20% sulfuric acid in ethanol followed by charring at ˜150° C. or by spraying with a solution of (NH4)6Mo7O24.H2O (25 g / L) in 10% sulfuric acid in ethanol followed by charring at ˜150° C. Column chromatography wa...
example iii
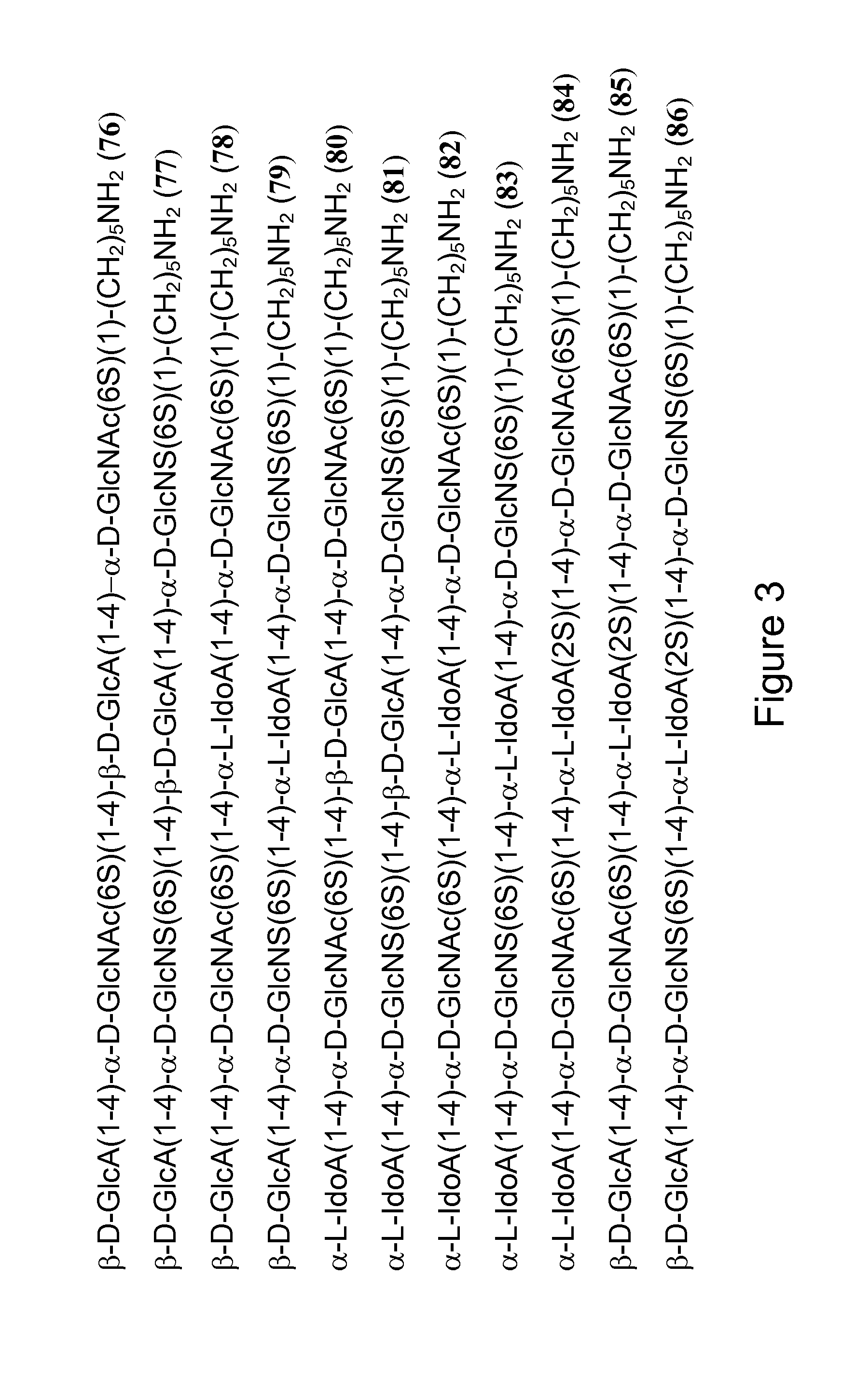
Synthesis and Use of Libraries of Heparan Sulfate Derivatives
Heparan Sulfates as Regulators of Protein Function
[0348]Heparan sulfate (HS) is required for many biological events and is structurally very diverse. Specific glycan sequences confer selective protein-binding properties to HS. However, ligand identification for specific GAG binding proteins is a major challenge in glycobiology.
[0349]Activities of HS include stabilizing proteins, restricting protein mobility and localization, altering protein conformation, and acting as a template for assembly of multi-protein complexes. Schematic representations of some of the activities of HS are shown in FIG. 5.
Chemical Synthesis of Oligosaccharides
[0350]Until now the chemical synthesis of a wide range of HS oligosaccharides has been an elusive goal. Anomeric control has been often problematic, the outcome of glycosylation has been unpredictable, and the preparation of glycosyl donor and acceptor molecules has proven very time consuming....
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption | aaaaa | aaaaa |
| enzyme activity | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com