Azepine fused ring derivatives as well as preparation method and application thereof
A technology for azapine and derivatives, applied in the field of azapine fused ring derivatives and their preparation, can solve the problems that the types cannot meet people’s needs, achieve rich diversity and product types, simple and fast preparation, and rapid build effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
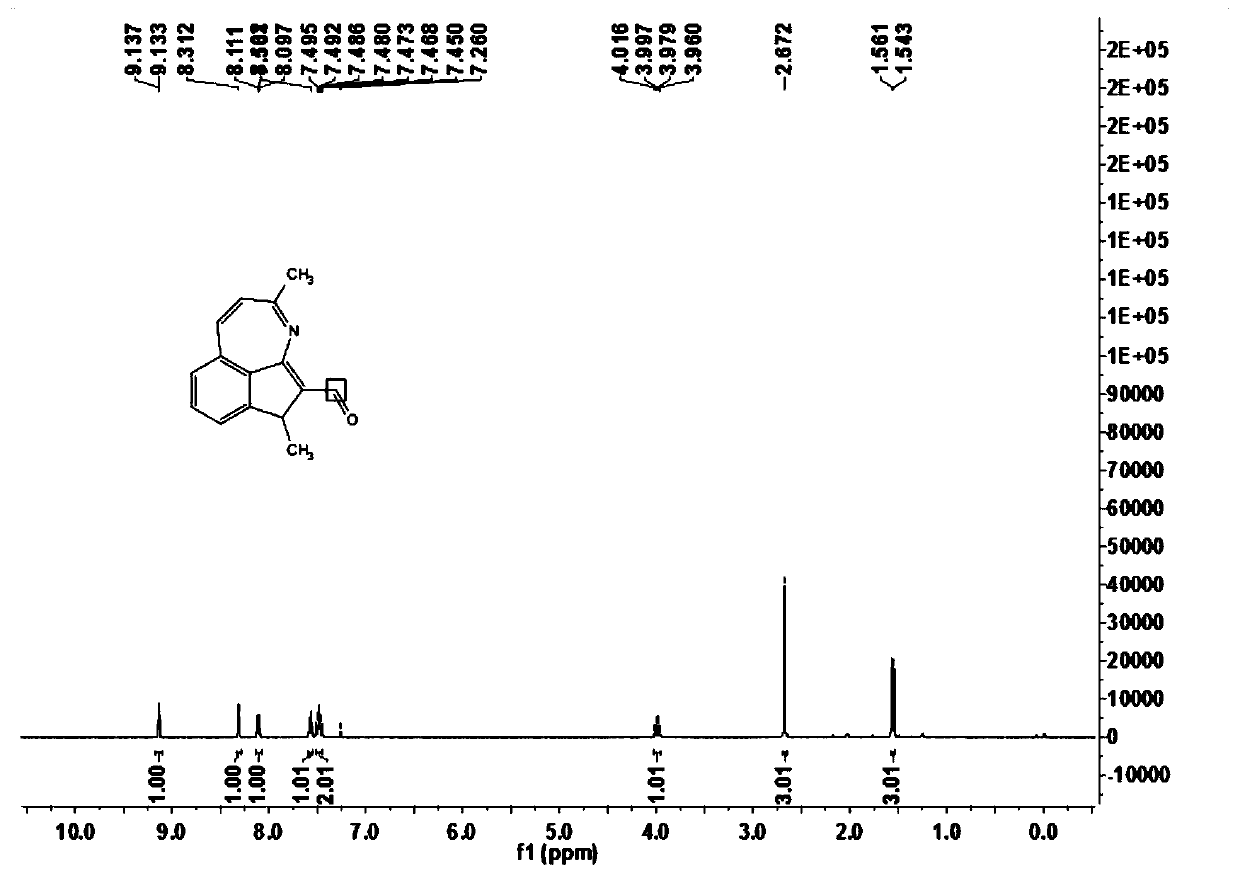
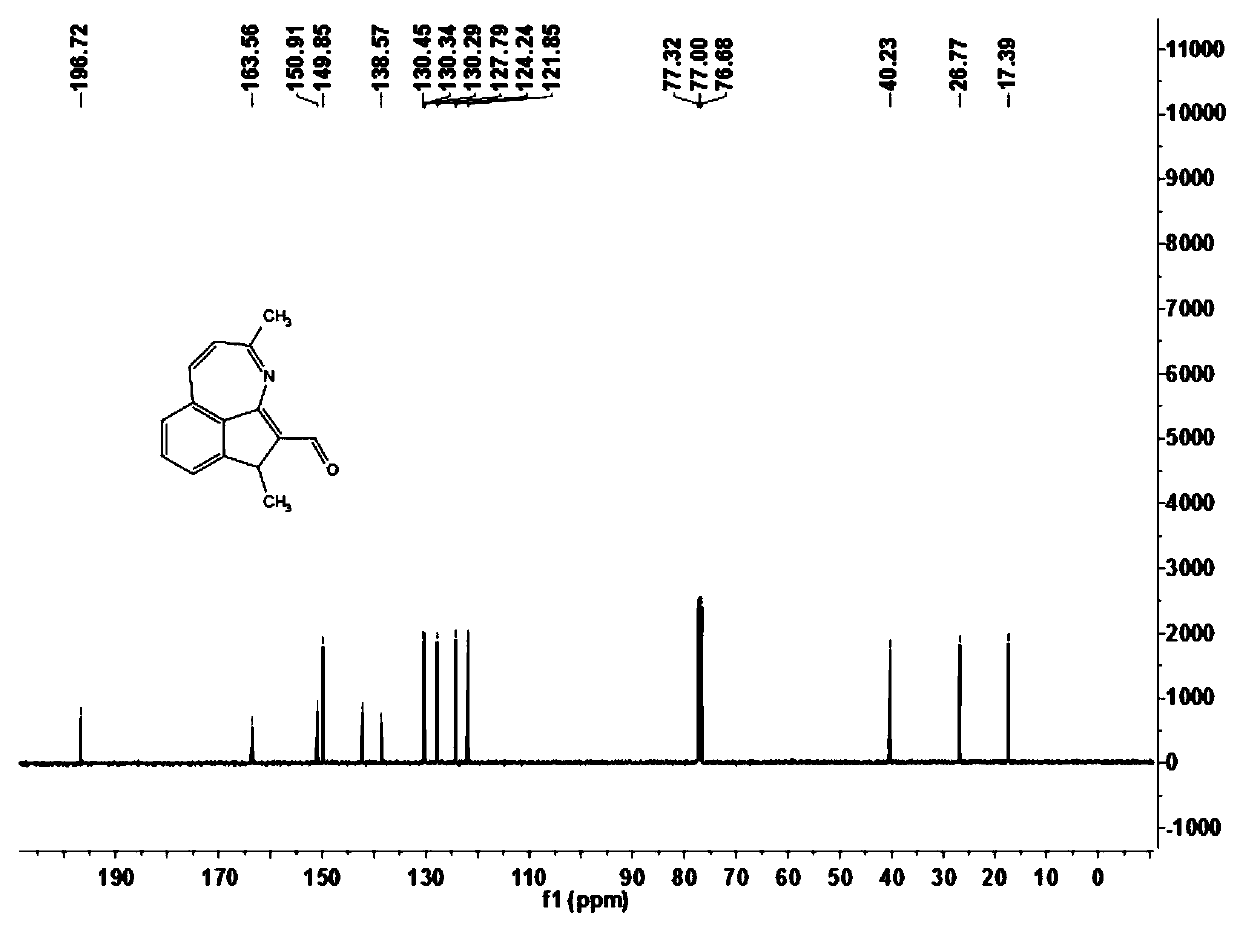
[0066] In this example, 3,6-dimethyl-6H-indeno[1,7-bc]aza-5-carbaldehyde (1a) was prepared, and its reaction formula is as follows:
[0067]
[0068] Under an atmosphere of atmospheric pressure air, add ethyl benzimidate compound 2a (30.0mg, 0.20mmol), crotonaldehyde 3a (24μL, 0.40mmol), trivalent rhodium catalyst [Cp*Rh (CH 3 EN) 3 ][SbF 6 ] 2 (0.002mmol), sodium acetate (5.0mg, 0.06mmol) and 1,2-dichloroethane (DCE, 1mL), at a temperature of 100 ° C for 3h for the first reaction, TLC detection of benziminoic acid ethyl After the ester compound 2a was reacted, butenone 4a (24 μL, 0.40 mmol) was added to carry out the second reaction for 9 hours. After the reaction was completed, it was cooled to room temperature, filtered through diatomaceous earth, and concentrated to obtain the crude product. The crude product was chromatographically separated on a prepared silica gel plate, and the selected developer or eluent was petroleum ether and ethyl acetate at a volume ratio ...
Embodiment 2
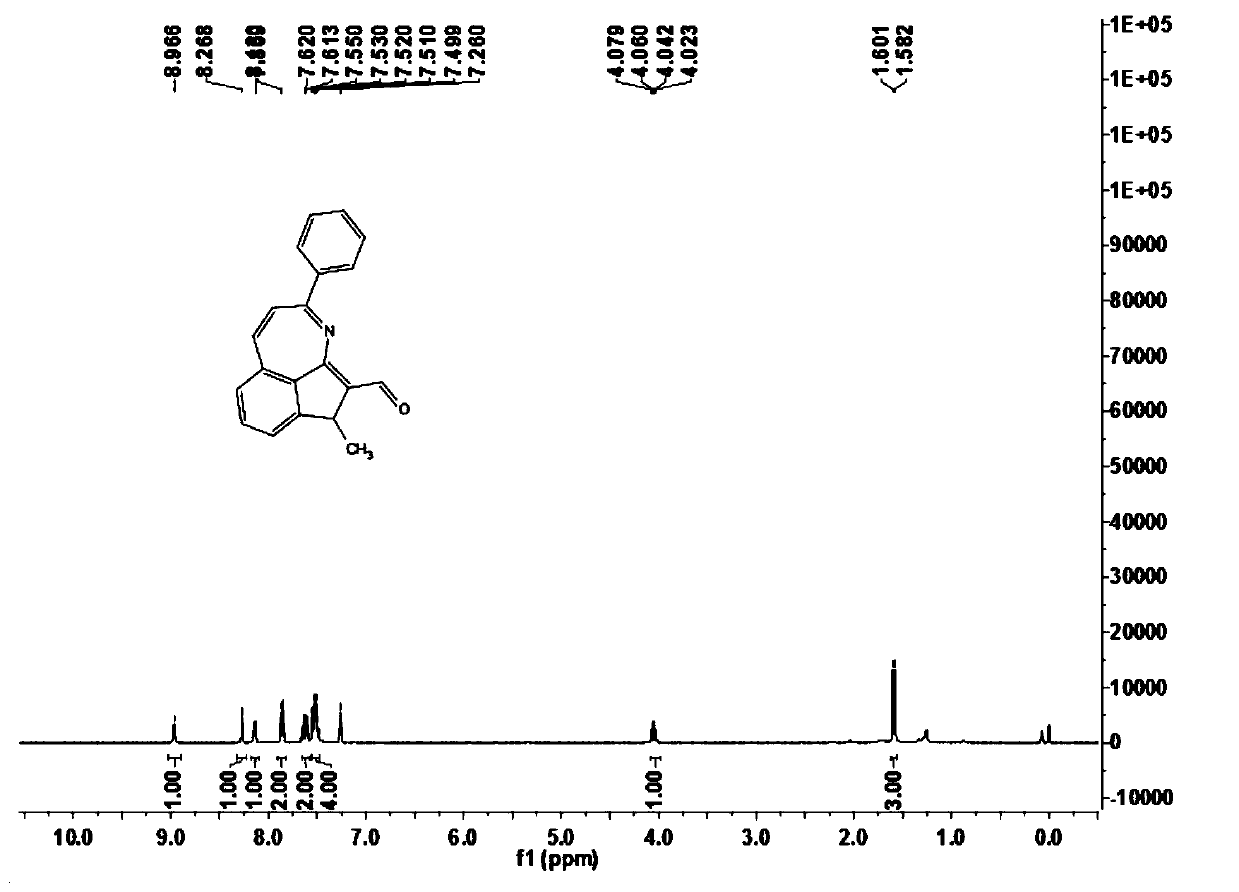
[0072] In this example, the preparation of 6-methyl-3-phenyl-6H-indeno[1,7-bc]aza-5-carbaldehyde (1b) is carried out, and its reaction formula is as follows:
[0073]
[0074] Under an atmospheric pressure air atmosphere, add ethyl benzimidate compound 2a (30.0mg, 0.20mmol), crotonaldehyde 3a (30μL, 0.50mmol), trivalent rhodium catalyst [Cp*RhCl 2 ] 2 (0.002mmol), silver trifluoromethanesulfonimide (0.006mmol), copper acetate (4mg, 0.02mmol), sodium acetate (1.60mg, 0.02mmol) and 1,2-dichloroethane (DCE, 1mL) , the first reaction was carried out at a temperature of 100°C for 3h, and after the reaction of ethyl benzimidate compound 2a was detected by TLC, butenone 4a (30μL, 0.50mmol) was added, and the second reaction was carried out for 9h. After completion, cool to room temperature, filter through diatomaceous earth, and concentrate to obtain a crude product. The crude product was separated by chromatography on the prepared silica gel plate, and the selected developer or...
Embodiment 3
[0077] In this example, the preparation of 1-(6-methyl-3-phenyl-6H-indeno[1,7-bc]azepine-5-yl)-ethanone (1c) is carried out, and its reaction formula is as follows :
[0078]
[0079] Under an atmosphere of atmospheric pressure air, add ethyl benzimidate compound 2a (30.0mg, 0.20mmol), 3-penten-2-one 3b (30μL, 0.50mmol), trivalent Rhodium catalyst [Cp*RhCl 2 ] 2 (0.004mmol), silver trifluoromethanesulfonimide (0.01mmol), copper acetate (4mg, 0.02mmol), sodium acetate (1.6mg, 0.02mmol) and 1,2-dichloroethane (DCE, 1mL) , the first reaction was carried out at a temperature of 100° C. for 3 h. After the reaction of ethyl benzimidate compound 2a was detected by TLC, phenyl enone 4b (30 μL, 0.50 mmol) was added, and the second reaction was carried out for 9 h. After the reaction was completed, it was cooled to room temperature, filtered through diatomaceous earth, and concentrated to obtain a crude product. The crude product was separated by chromatography on a prepared sili...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com