Therapeutic agent for autoimmune diseases or allergy, and method for screening for the therapeutic agent
a technology for autoimmune diseases or allergies, applied in the field of pharmaceutical composition, can solve problems such as tissue damage and direct damage to tissues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Methods
Mice
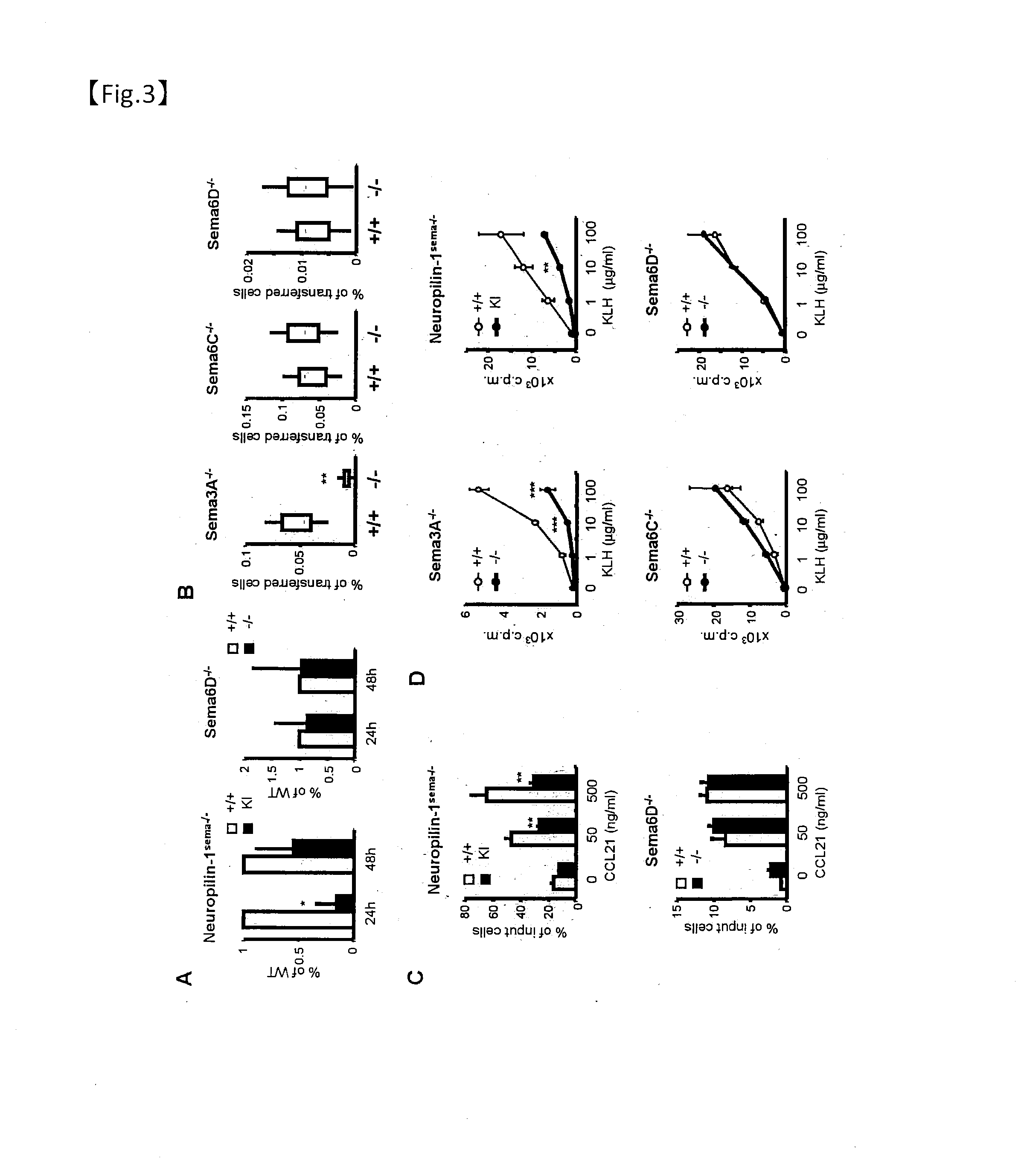
[0220]Plexin-A1− / −, Sema3K− / −, Sematic− / − and NP-1 knock-in mice with a C57BL / 6 background have already been established. OT-IITg mice were provided by Mr. W. R. Heath.
[0221]For the Sema6D− / −, a 0.6 kbp fragment comprising the second exon with start codon and the third exon were replaced with a neo resistance cassette, and a herpes simplex virus thymidine kinase (HSY-TK) gene was inserted for selecting a targeting vector from random integration. Linearized targeting plasmid DNA was transfected into embryonic stem (ES) cells by electroporation. After double selection with G418 and ganciclovir, 320 resistant clones were screened for homologous recombination of Sema6D alleles by PCR and Southern blot analysis. For the Southern blot analysis, genome DNA was isolated from wild-type and Sema6D-target ES cells and digested with EcoRI, and separated by agarose gel electrophoresis. The DNA was transferred to a nylon blotting membrane (HybondN) in accordance with the manufacturer's...
example 2
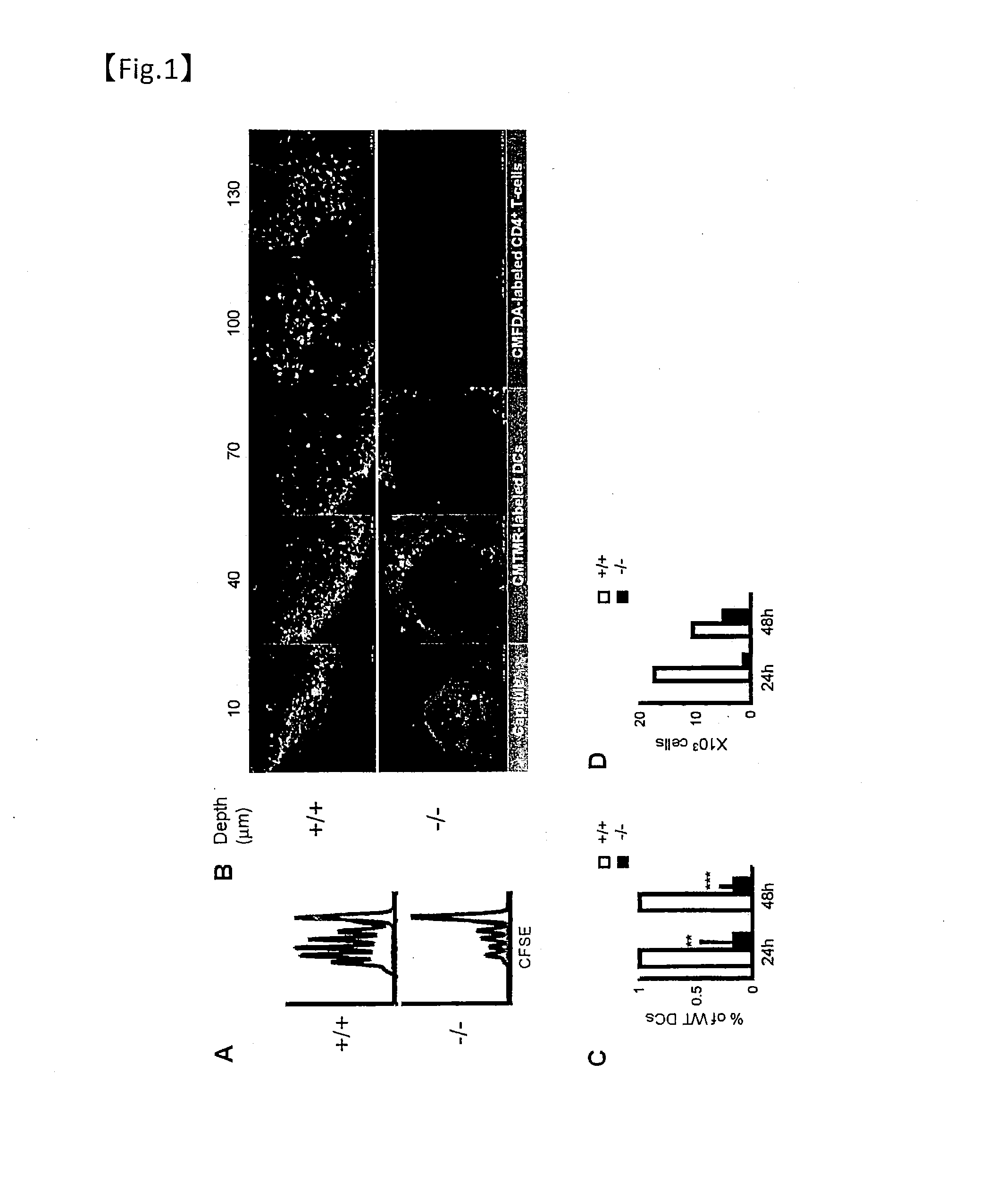
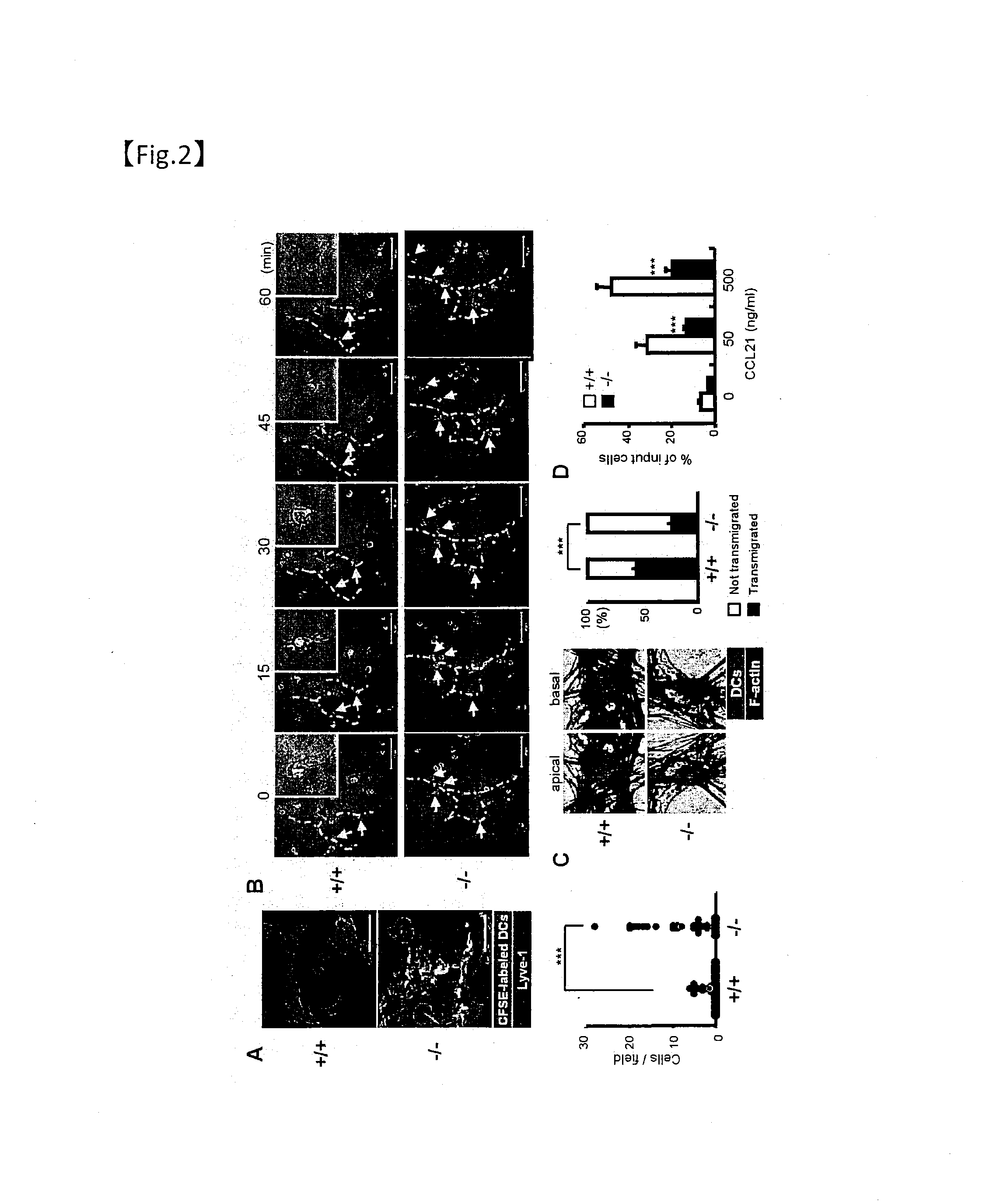
[0302]To evaluate the role of Plexin-A1 in cell migration in vivo, dendritic cells collected from wild-type or Plexin-A1 knockout mice and labeled with CFSE were adoptively transferred into the dermis of mice treated with oxazolone. The behavior of the transferred dendritic cells was then monitored.
[0303]Adoptive transfer was performed by the following methods. Dendritic cells from bone marrow were labeled for 10 minutes at 22° C. in 5 μl of CFSE (carboxyfluorescein diacetate succinimidyl ester). The cells were then washed with PBS. 1×106 dendritic cells suspended in PBS were subcutaneously injected into the foot pads of recipient mice. 24 and 48 hours after injection, the popliteal lymph nodes were collected. The lymph nodes were treated for 30 minutes at 37° C. with 1 mg / ml of collagenase D. The cell numbers were counted and analyzed by flow cytometry. As described in Cavanagh et al. (Nat. Immunol. (2005) 6, 1029-1037), the percentage of migrating dendritic cells corresponds to th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com