Novel live recombinant booster vaccine against tuberculosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Materials and Methods
[0042]A. BCG Strain (Wild-type M. bovis BCG Tice)
[0043]This strain was maintained in 7H9 medium pH 6.7 (Difco) at 37° C. in a 5% CO2-95% air atmosphere as unshaken cultures. Cultures were sonicated once or twice weekly for 5 min in a sonicating water bath to reduce bacterial clumping, as described (see, e.g. Horwitz, et al. (2000). “Recombinant bacillus calmette-guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model.”Proc Natl Acad Sci USA 97(25): 13853-8).
B. Recombinant Attenuated Listeria monocytogenes Vaccines
1. rLm / Mtb30(01)
[0044]a. Construction of rLm / Mtb30(01)
[0045]rLm / Mtb30(01), an attenuated recombinant Listeria monocytogenes expressing the M. tuberculosis 30 kDa major secretory protein (Mtb30), was constructed using the attenuated L. monocytogenes host strain, LmΔactA, an L. monocytogenes str...
experiment 1
Immunogenicity of rLm / Mtb30 in Prime-Boost Vaccination Regimen
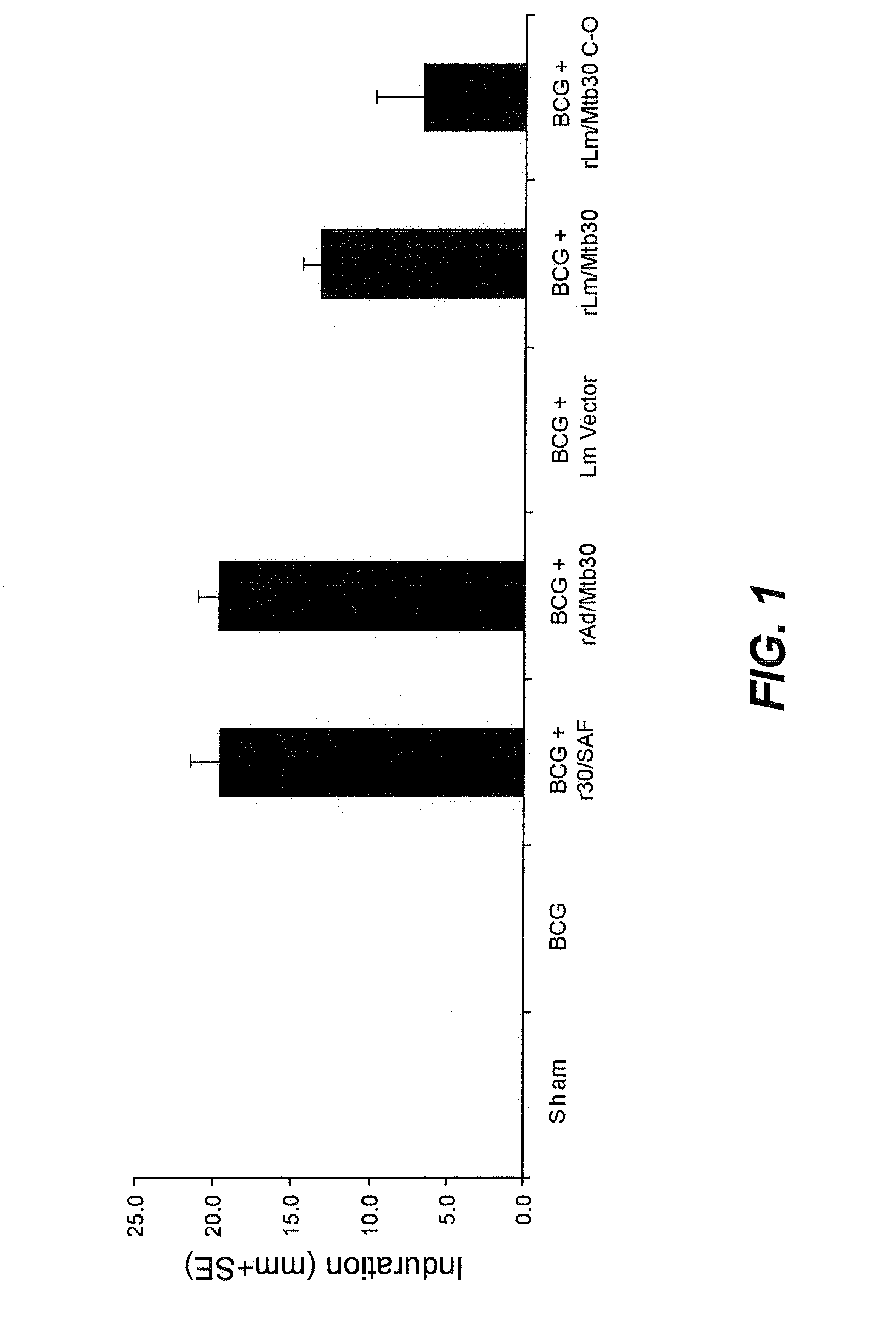
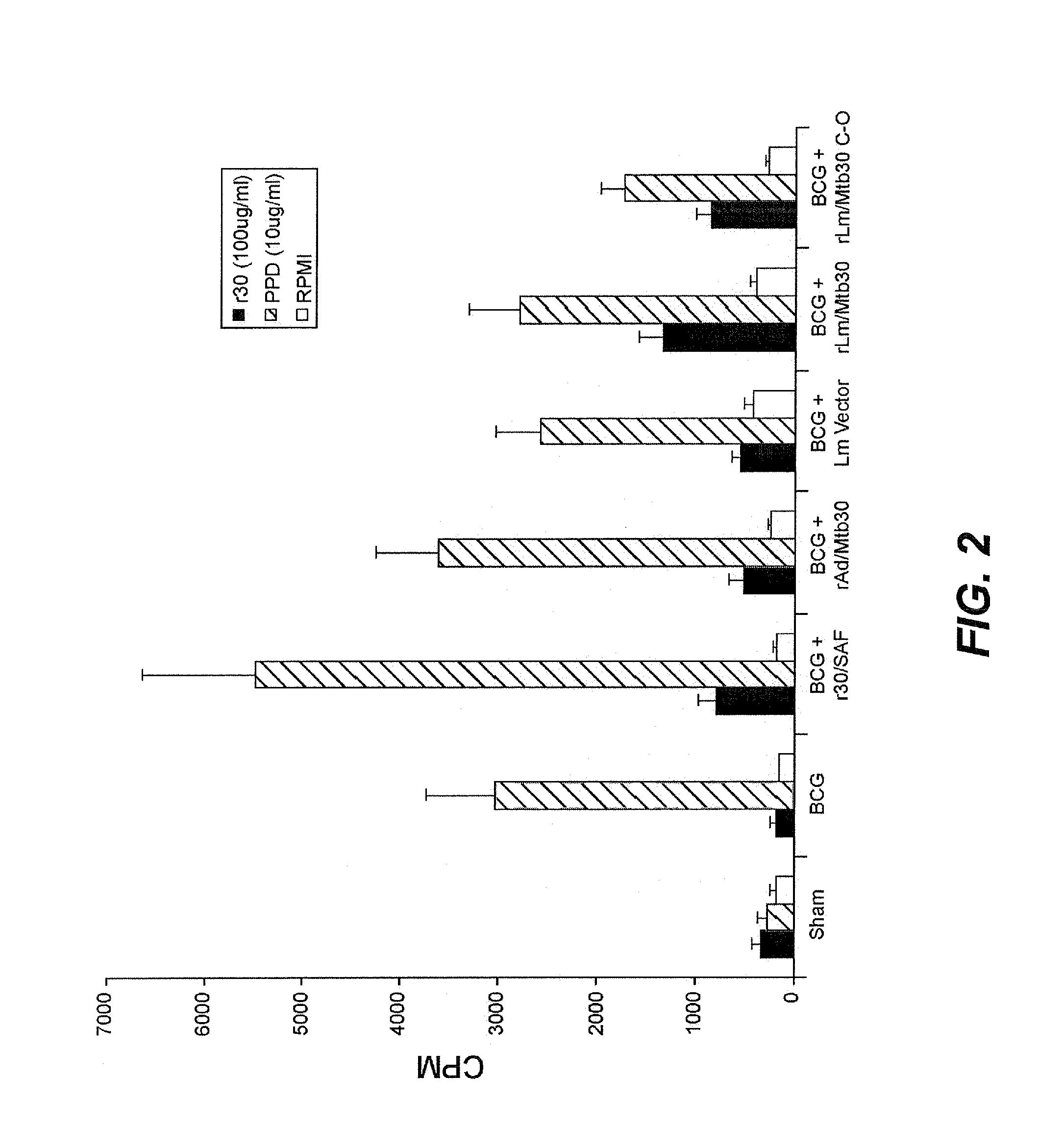
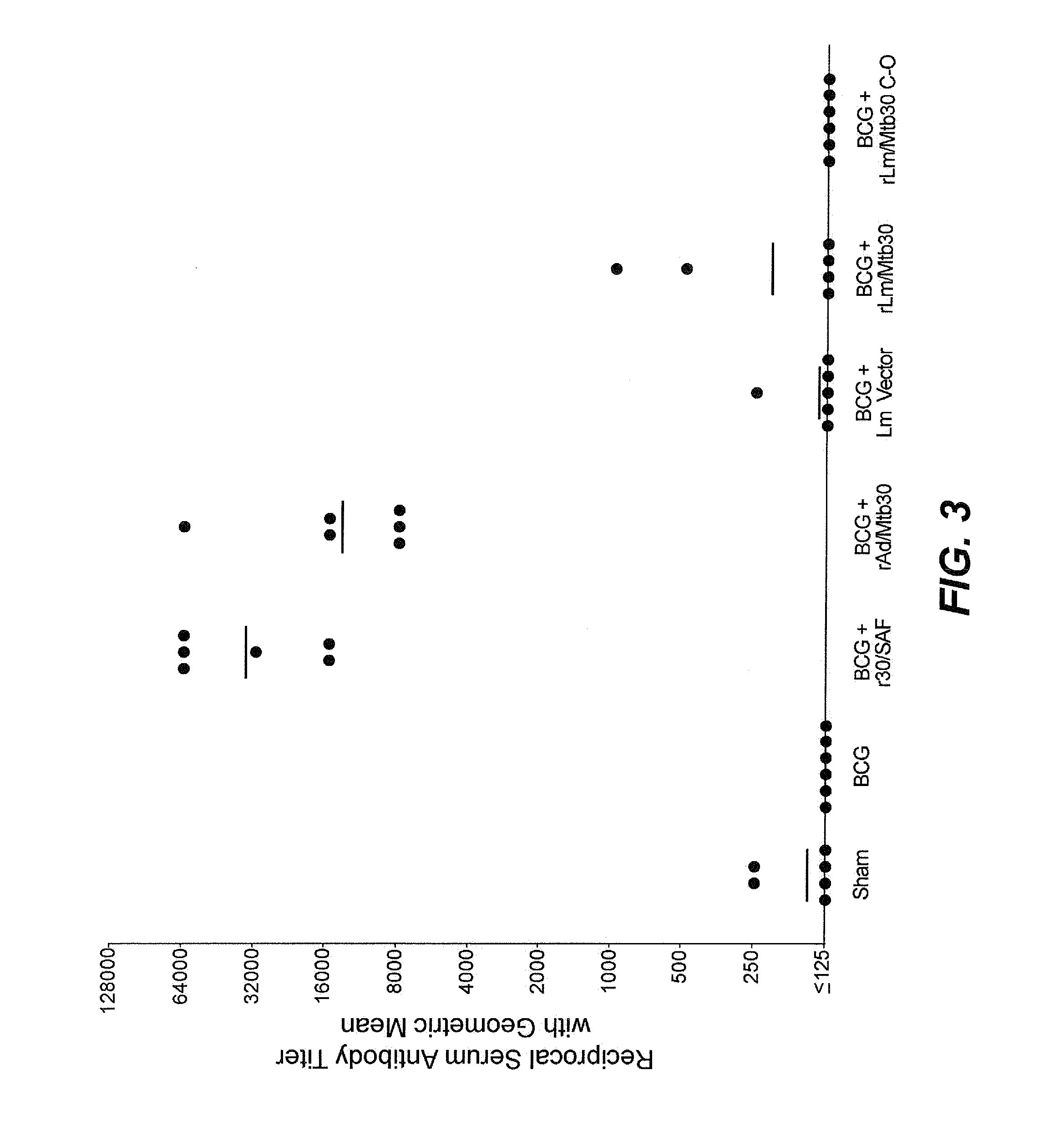
[0071]Specific-pathogen free 250-300 g outbred male Hartley strain guinea pigs from Charles River Breeding Laboratories, in groups of 6, were immunized intradermally as follows:[0072]Group A: Sham[0073]Group B: BCG Tice Parental Control (103 CFU) at Week 0[0074]Group C: BCG Tice Parental Control (103 CFU) at Week 0 and 100 μg of r30 in SAF adjuvant at Week 4[0075]Group D: BCG Tice Parental Control (103 CFU) at Week 0 and rAd / Mtb30 at Week 4[0076]Group E: BCG Tice Parental Control (103 CFU) at Week 0 and Lm Empty Vector at Week 4[0077]Group F: BCG Tice Parental Control (103 CFU) at Week 0 and rLm / Mtb30 at Week 4[0078]Group G: BCG Tice Parental Control (103 CFU) at Week 0 and rLm / Mtb30 C-0 at Week 4
[0079]At week 8, animals were tested for cutaneous delayed-type hypersensitivity (c-DTH) to r30, and after the skin test was assessed, the animals were euthanized for assay of splenic lymphocyte proliferation and antibody respons...
experiment 2
Protective Efficacy of rLm / Mtb30 in a Prime-Boost Vaccination Regimen in Guinea Pigs
[0087]Specific-pathogen free 250-300 g outbred male Hartley strain guinea pigs from Charles River Breeding Laboratories, in groups of 15 (except for the sham group, which had 9 animals), were immunized intradermally as follows:[0088]Group A: Sham[0089]Group B: BCG Tice Parental Control (105 CFU) at Week 0[0090]Group C: BCG Tice Parental Control (105 CFU) at Week 0 and 100 μg of r30 in SAF adjuvant at Week 4[0091]Group D: BCG Tice Parental Control (105 CFU) at Week 0 and 100 μg of r30 in SAF adjuvant at Weeks 4 and 8[0092]Group E: BCG Tice Parental Control (105 CFU) at Week 0 and rAd / Mtb30 at Week 4[0093]Group F: BCG Tice Parental Control (105 CFU) at Week 0 and rAd / Mtb30 at Weeks 4 and 8[0094]Group G: BCG Tice Parental Control (105 CFU) at Week 0 and rLm / Mtb30 at Week 4[0095]Group H: BCG Tice Parental Control (105 CFU) at Week 0 and rLm / Mtb30 at Weeks 4 and 8
[0096]Twenty weeks after immunization, all...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electric charge | aaaaa | aaaaa |
| Current | aaaaa | aaaaa |
| Digital information | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com