Processes using vlps with capsids resistant to hydrolases
a technology of hydrolase and capsids, which is applied in the field of viruslike particles, can solve the problems of high separation efficiency of recombinant proteins using these simple isolation processes, unique and complex isolation process, and limited application to simple systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0108]The following non-limiting examples are included to illustrate various aspects of the present disclosure. It will be appreciated by those of skill in the art that the techniques disclosed in the following examples represent techniques discovered by the Applicants to function well in the practice of the invention, and thus can be considered to constitute preferred modes for its practice. However, those of skill in the art should, in light of the instant disclosure, appreciate that many changes can be made in the specific examples described, while still obtaining like or similar results, without departing from the scope of the invention. Thus, the examples are exemplary only and should not be construed to limit the invention in any way. To the extent necessary to enable and describe the instant invention, all references cited are herein incorporated by reference.
example a
Propagation of MS2 Bacteriophage
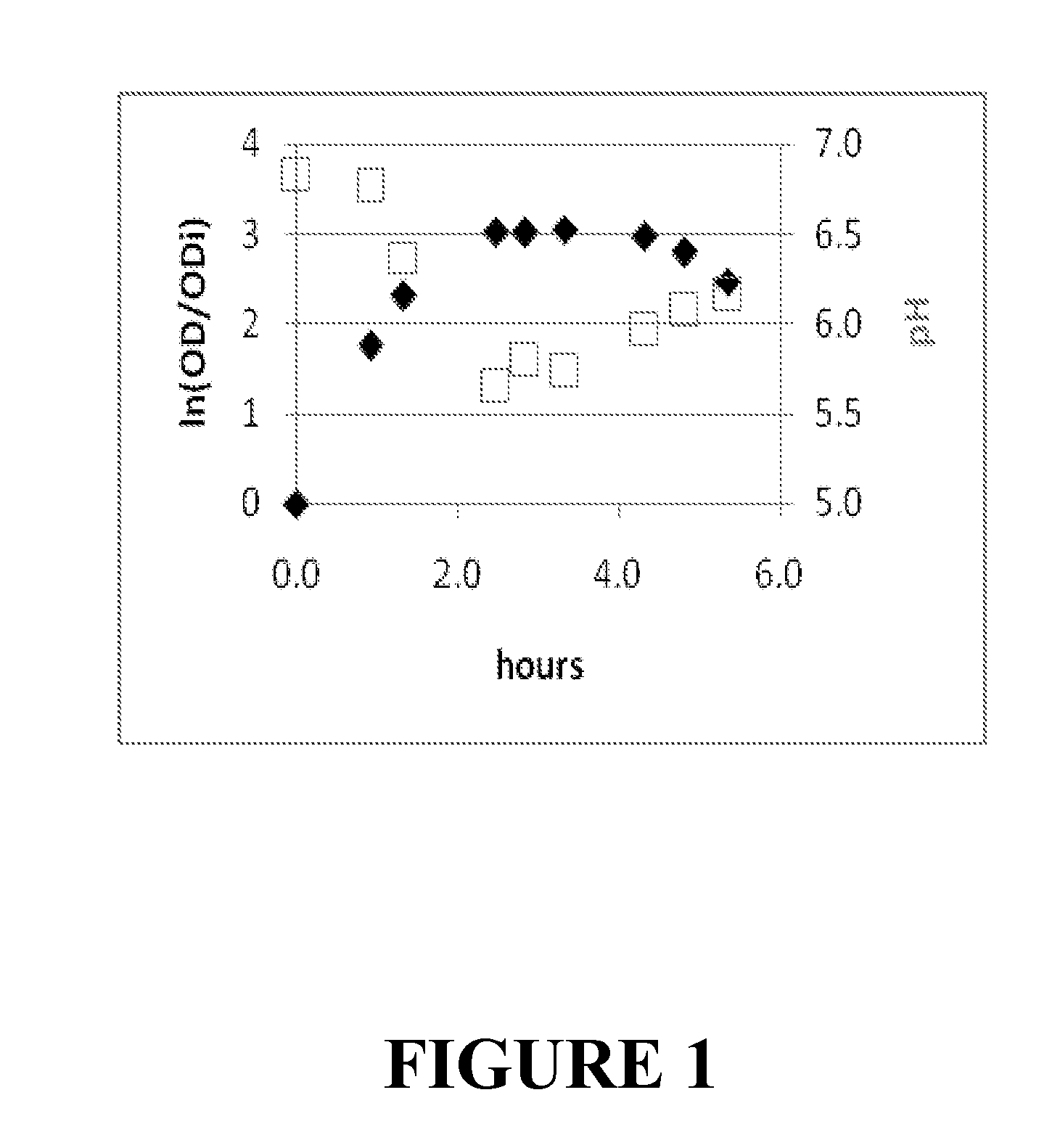
[0109]MS2 bacteriophage (ATCC No. 15597-B1, from American Type Culture Collection, Rockville, Md.) and its E. Coli host (ATCC No. 15669) were obtained from ATCC and propagated using the procedure described by Strauss and Sinsheimer (1963) J. Mol. Biol. 7:43-54 J. Mol. Biol. 7:43-54. Results are plotted in FIG. 1. Optical Density (OD) at 600 nm and pH were followed during the reaction. ODi represents OD immediately after inoculation with host. Infection was done at 2.3 hours. Ln(OD / ODi) was plotted on the left axis (full diamonds) and pH was plotted on the right axis (open squares). This experiment was ended 5.3 hours after inoculation with host. Lysate obtained was centrifuged at 2,000 g and filtered through a 0.2 μm membrane to eliminate remaining bacteria and bacterial debris.
example b
Purification of MS2 Bacteriophage Using Proteinase K and Ultrafiltration
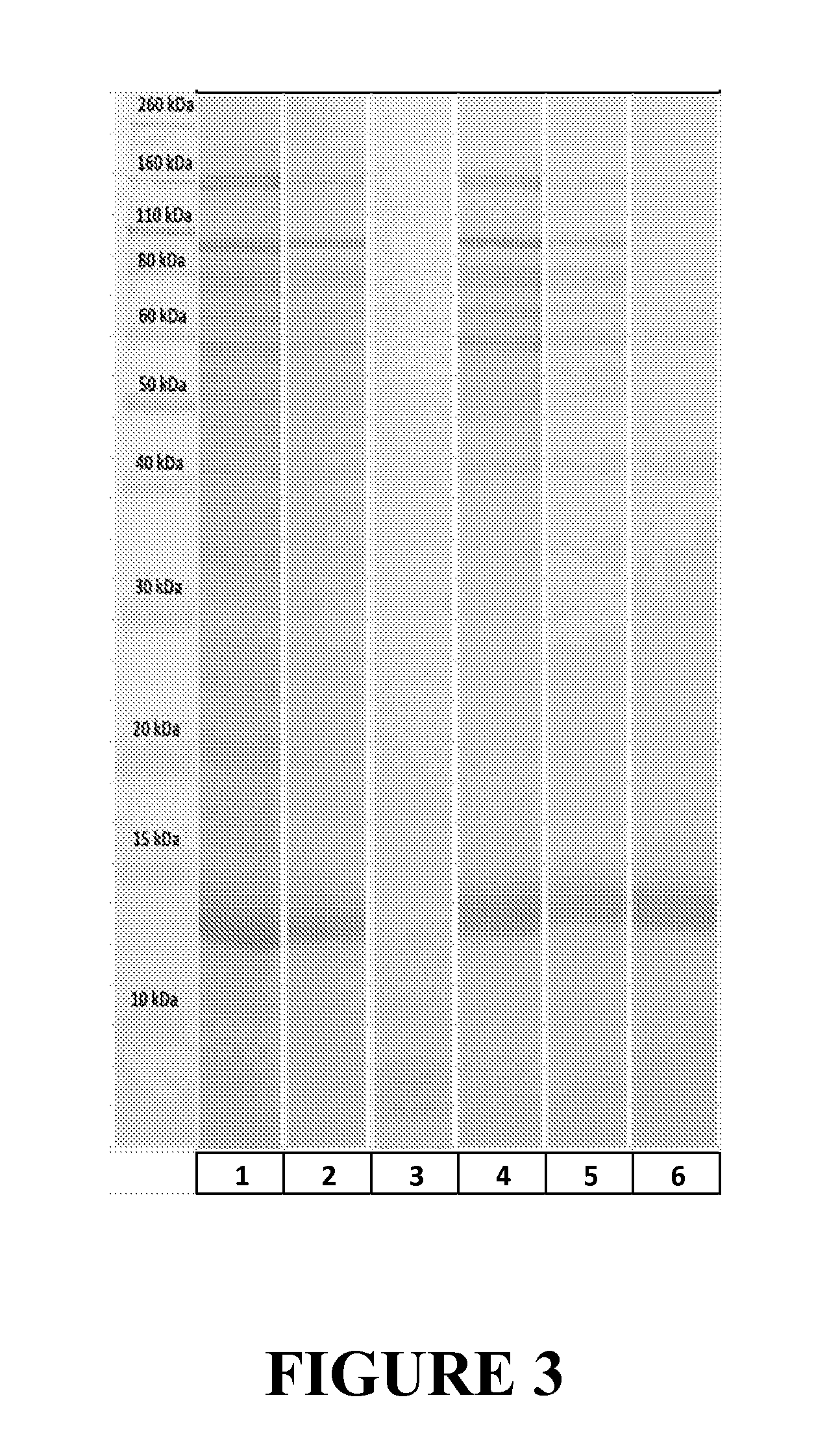
[0110]Purification of MS2 bacteriophage was conducted as follows. Samples were taken during purification and SDS PAGE analysis was run on the samples. Results obtained are shown in FIG. 2.
8 mL lysate obtained at end of Example A (sample in Lane 1, FIG. 2) was filtered through a 300 kDa membrane (Vivaspin 2, from Sartorius Stedim, Bohemia, N.Y.) and the filtrate was filtered through a 100 kDa membrane, from which 1 mL of retentate was obtained (sample in Lane 2, FIG. 2). This retentate was divided in two equal parts. To one half (control) 2064, mM CaCl2 aqueous solution at pH=7.5 was added. To the second half (Proteinase) 0.15 mg Proteinase K (Sigma Aldrich, St. Louis, Mo.) dissolved in 2064, 20 mM CaCl2 aqueous solution at pH=7.5 and was added. Both tubes were incubated at 37° C. and after 1 hour they were placed in an ice-water bath. Samples were then taken and analyzed: control sample in Lane 3, FIG. 2, and Pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com