Anti-heparan sulfate peptides that block herpes simplex virus infection in vivo
a technology of anti-heparan sulfate and peptides, which is applied in the direction of peptides/protein ingredients, peptides, immunoglobulins, etc., can solve the problems of inability to cure the subject of infection, the severity of hsv outbreak, and the severity of infection, so as to inhibit viral infection and/or receptor-mediated cell-to-cell fusion, and strong herpesvirus entry-inhibiting activities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Experimental Procedures
[0125]Selection of Phages Against HS and 3-OS HS by Library Panning
[0126]A phage display library (PhD™-12) expressing 12-mer peptides fused to a minor coat protein (pIII) of a non-lytic bacteriophage (M13) was purchased from New England Biolabs (Cambridge, Mass.). A purified form of HS isolated from bovine kidney was purchased from Sigma. Soluble 3OS HS modified by 3-OST-3 was prepared as previously described. Tiwari et al., J. Gen. Virol. 88:1075-1079 (2007).
[0127]Screening of the phage display library was accomplished by an affinity selection (or bio-panning) process during which phage populations were selected for their ability to bind HS and 3OS HS (modified by 3-OST-3). Both targets at a concentration of 10 μg / ml were used for overnight coating of wells of 96 well plates (Nalge Nunc International, Naperville, Ill.) in a humidifier chamber at 4° C. The following day, the plates were blocked for 1 hr at room temperature with 5 mg / ml bovine serum albumin (BS...
example 2
Identification of HS and 3-OS HS Binding Peptides that Block HSV-1 Entry
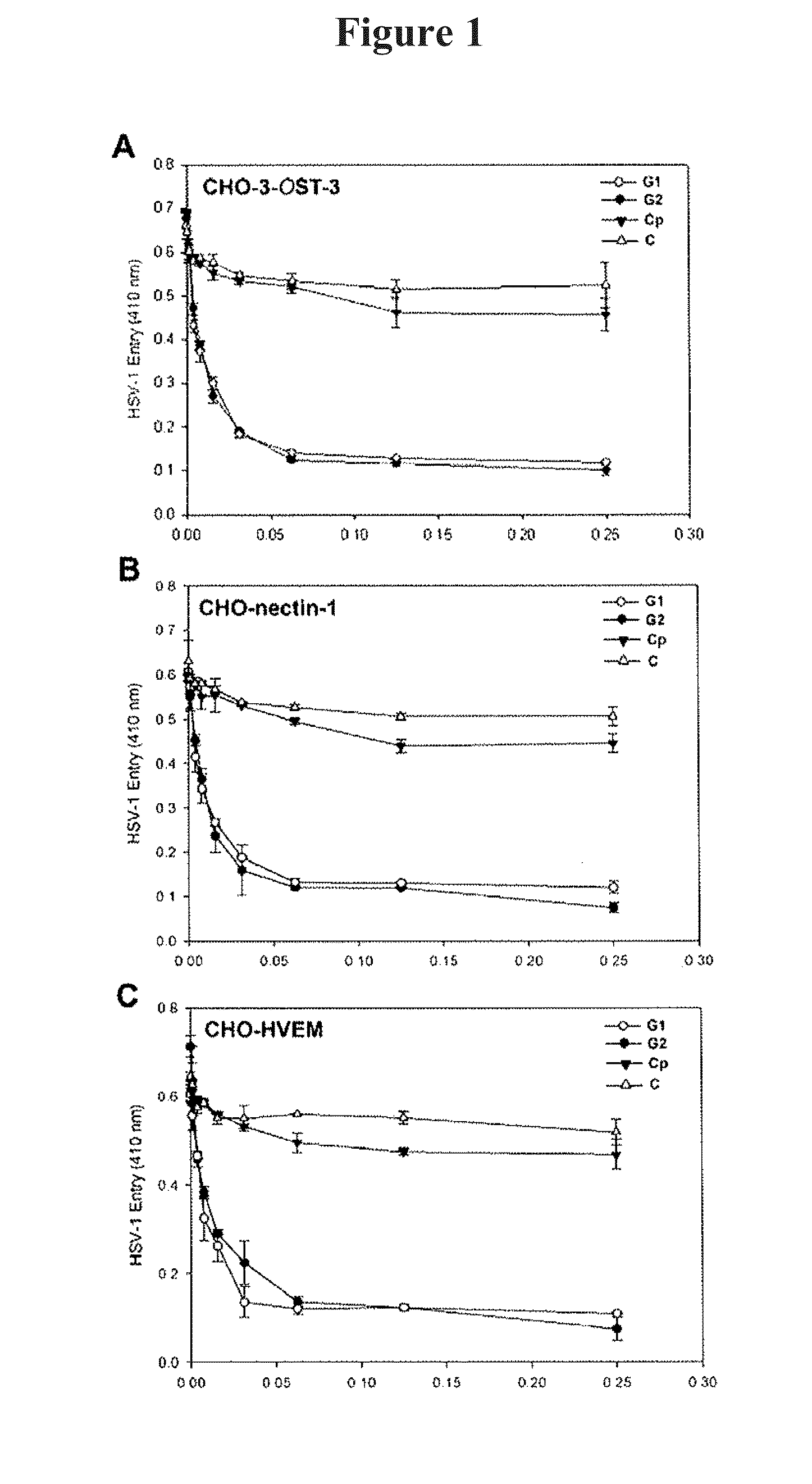
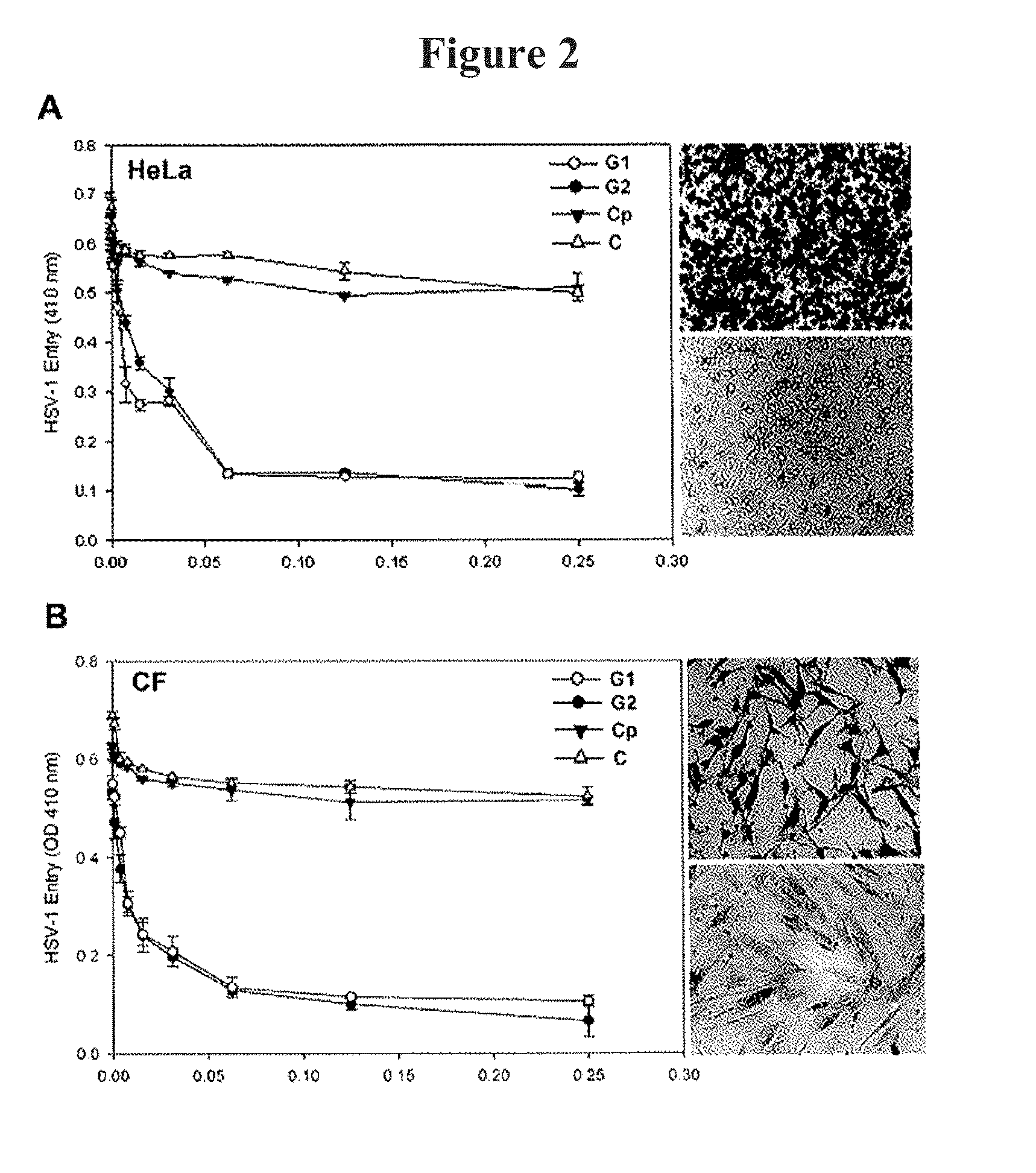
[0150]Three rounds of screening of phages from a 12-mer peptide phage display library resulted in the enrichment of phages that specifically-bound to HS and / or to 3-OS HS. Peptide sequences from individual plaques were deduced by determining the nucleotide sequences of the portion of the phage genome that encoded them. The predicted peptide sequences of about 200 plaques were determined and sorted into two groups on the basis of their targets. A frequently repeating peptide sequence from each group was subsequently selected for further characterization. The two most frequently isolated peptide sequences LRSRTKIIRIRH (designated G1 for HS binding group 1) and MPRRRRIRRRQK (designated G2 for 3-OS HS binding group 2) were synthesized and examined for each peptide's ability to inhibit HSV-1 infection of 3-OST-3 (FIG. 1A), nectin-1 (FIG. 1B), and HVEM (FIG. 1C) expressing CHO-K1 cells. Cells were pre-incubated with G...
example 3
The Peptide Inhibitors are Also Active Against a Variety of HSV-1 Strains
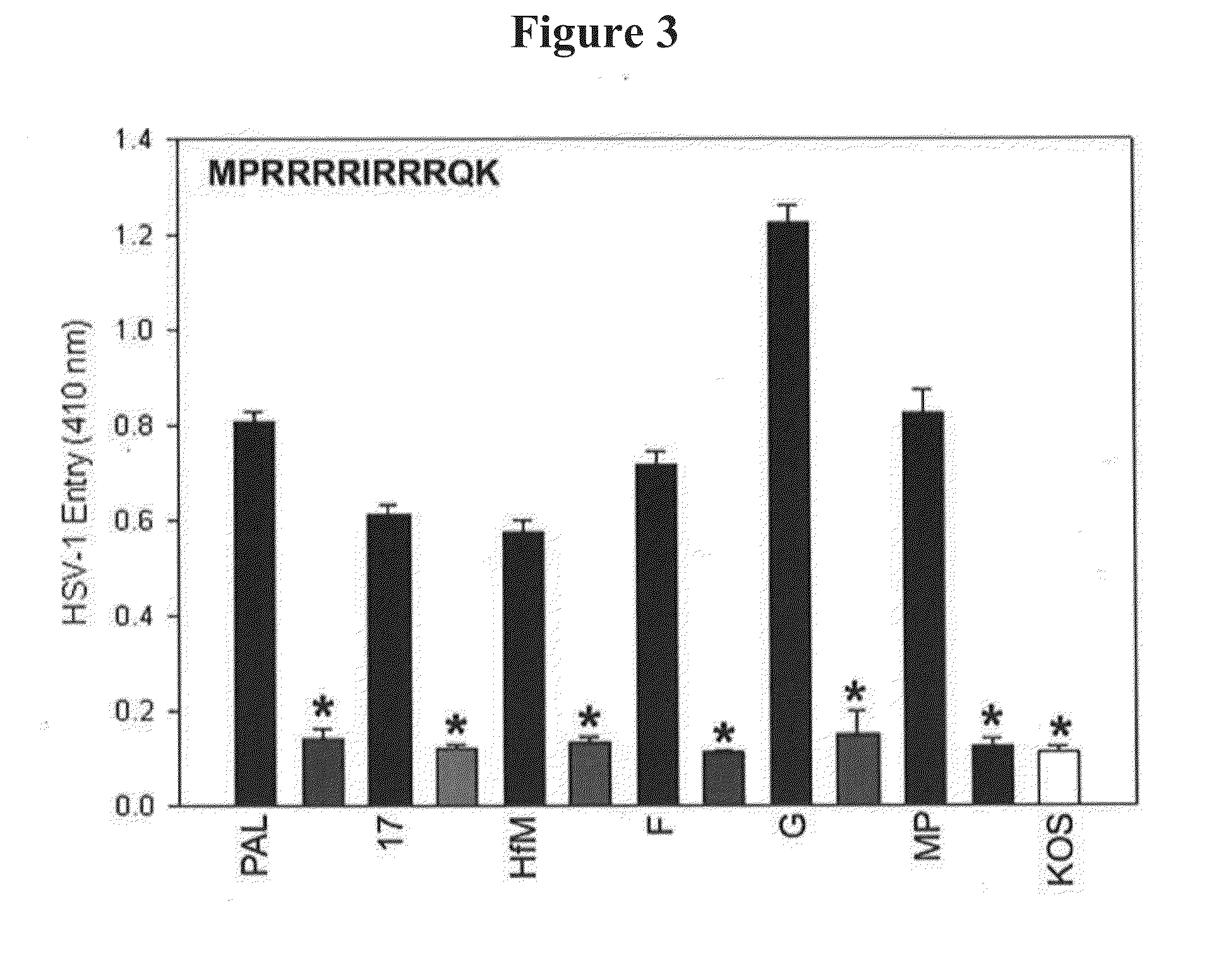
[0154]This Example demonstrates that the inhibitory effect of the G2 peptide is not limited by viral strain or serotype.
[0155]The anti-HSV properties of the G1 and G2 peptides were evaluated against common strains of HSV-1 and HSV-2 (i.e., strains F, G, Hfem, MP, KOS, and 17). Dean et al., Virology 199:67-80 (1994). 3-OST-3 expressing CHO Ig8 reporter cells were used that express β-galactosidase upon viral entry. Montgomery et al., Cell 87:427-436 (1996). Cells were pre-incubated with G1, G2, or control peptide (Cp) and subsequently infected with the viruses. G2 or Cp control at 0.5 mM concentration was incubated for 60 min at room temperature with a reporter CHO-Ig8 cells that express β-galactosidase upon HSV-1 entry. After incubation, the cells were infected with HSV-1 (Pal, 17, Hfm, F, KOS, and MP) and HSV-2 (G) strains at 25 pfu / cell for 6 hr at 37° C. Blockage of viral entry was measured by ONPG assay as d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Acidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com