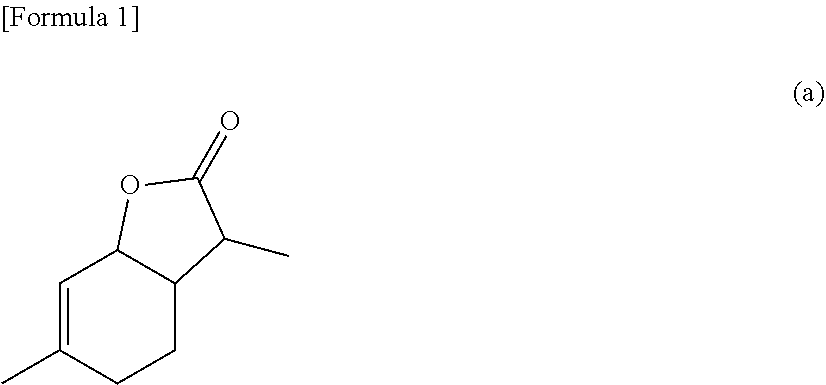Process for producing wine lactone
a technology of wine lactone and process, applied in the field of process for producing wine lactone, can solve the problems of not being suitable for industrial use, not cost-effective for producing all stereoisomers, and difficult to use process starting from limonene on an industrial scal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
1. First Embodiment
[0065]The production process according to the first embodiment of the present invention is characterized by comprising step A), step B-1) and step C):
[wherein R1 is an alkyl group containing 1 to 4 carbon atoms, R2 is an alkyl group containing 1 to 4 carbon atoms, and X is a chlorine atom or a bromine atom].
[0066]Detailed explanation will be given below for each step.
(1) Step A)
[0067]Step A) is intended to react a β-keto ester represented by formula (1):
[wherein R1 is an alkyl group containing 1 to 4 carbon atoms]
with a 2-halo ester represented by formula (2):
[wherein R2 is an alkyl group containing 1 to 4 carbon atoms, and X is a chlorine atom or a bromine atom]
under basic conditions to thereby obtain a 2-aceto-3-methyl-succinic acid ester represented by formula (3):
[wherein R1 is as defined in formula (1), and R2 is as defined in formula (2)].
[0068]In the above formulae, R1 and R2, which may be the same or different, are each an alkyl group containing 1 to 4 car...
second embodiment
2. Second Embodiment
[0209]The production process according to the second embodiment of the present invention comprises step A), step B-2), step B-3) and step C):
[wherein R1 is an alkyl group containing 1 to 4 carbon atoms, R2 is an alkyl group containing 1 to 4 carbon atoms, and X is a chlorine atom or a bromine atom].
[0210]Among the above steps in the second embodiment, step A) and step C) are the same as those of the first embodiment and their explanation will be omitted. Detailed explanation will be given below for step B-2) and step B-3).
(1) Step B-2)
[0211]Step B-2) is intended to react the 2-aceto-3-methyl-succinic acid ester obtained in step A) with methyl vinyl ketone under basic conditions, followed by decarboxylation reaction to thereby obtain an α-methyl-γ-keto acid ester represented by formula (5):
[wherein R2 is as defined in formula (2)].
[0212]Methyl vinyl ketone used in this reaction may be either a commercially available product or a synthetic product, as described abo...
third embodiment
3. Third Embodiment
[0236]The production process according to the third embodiment of the present invention comprises step A), step B-2) and step E):
[wherein R1 is an alkyl group containing 1 to 4 carbon atoms, R2 is an alkyl group containing 1 to 4 carbon atoms, and X is a chlorine atom or a bromine atom].
[0237]Among the above steps in the third embodiment, step A) is the same as that of the first embodiment, while step B-2) is the same as that of the second embodiment. Thus, their explanation will be omitted. Detailed explanation will be given below for step E).
(1) Step E)
[0238]In a first case, step E) is intended for reduction reaction of the α-methyl-γ-keto acid ester obtained in step B-2) in the presence of a ruthenium complex selected from compounds represented by formula (6) or (7) and in the presence of a hydrogen donor to thereby obtain a compound represented by formula (a):
[0239]The ruthenium complexes represented by formulae (6) and (7) may be the same as those explained i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com