Therapeutic Compositions For Treatment Of Inflammation Of Ocular And Adnexal Tissues
a technology therapeutic compositions, which is applied in the field of ophthalmology, can solve the problems of systemic inhibition of signaling within the immune system, insufficient long-term persistence at the target, and the difficulty of using systemic drugs, and achieve the effect of reducing or preventing inflammation of ocular and adnexal tissues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
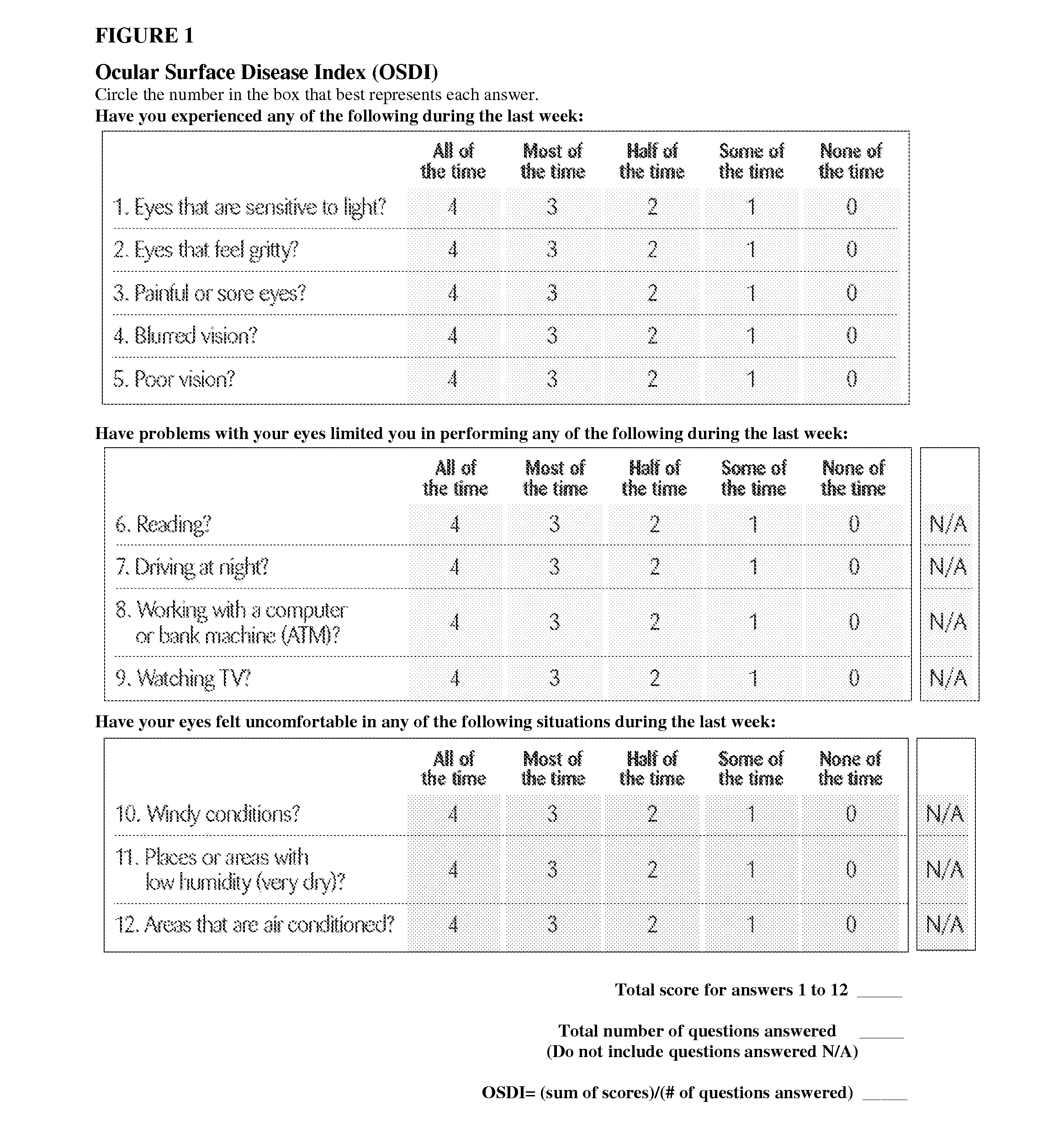
The Ocular Surface Disease Index (OSDI)
[0151]The Ocular Surface Disease Index (OSDI) is a 12-item questionnaire that provides a rapid assessment of the symptoms of ocular irritation consistent with ocular surface disease, including posterior blepharitis and dry eye disease, and their impact on vision-related functioning (FIG. 1). The 12 items of the OSDI questionnaire are graded on a scale of 0 to 4, where 0 indicates none of the time; 1, some of the time; 2, half of the time; 3, most of the time; and 4, all of the time. The total OSDI score is then calculated on the basis of the following formula: OSDI=[sum of scores for all questions answered)×100] / [(total number of questions answered)×4]. Thus, the OSDI is scored on a scale of 0 to 100, with higher scores representing greater disability. A negative change from baseline indicates an improvement in vision-related function and the ocular inflammatory disorders described herein. For the therapeutic method described herein, treatment ...
example 2
Tear Film Break-Up Time
[0153]The standard TBUT measurement is performed by moistening a fluorescein strip with sterile non-preserved saline and applying it to the inferior tarsal conjunctiva. After several blinks, the tear film is examined using a broad beam of the slit lamp with a blue filter. The time lapse between the last blink and the appearance of the first randomly distributed dark discontinuity in the fluorescein stained tear film is measured three times and the mean value of the measurements is calculated. The tear break-up time is evaluated prior to the instillation of any eye drops and before the eyelids are manipulated in any way. Break-up times less than 10 seconds are considered abnormal. A positive change from baseline indicates improvement in symptoms of the ocular inflammatory disorders described herein. The treatment described herein, leads to an improvement in TBUT significantly greater than that observed from treatment with vehicle alone.
example 3
Corneal and Conjunctival Staining
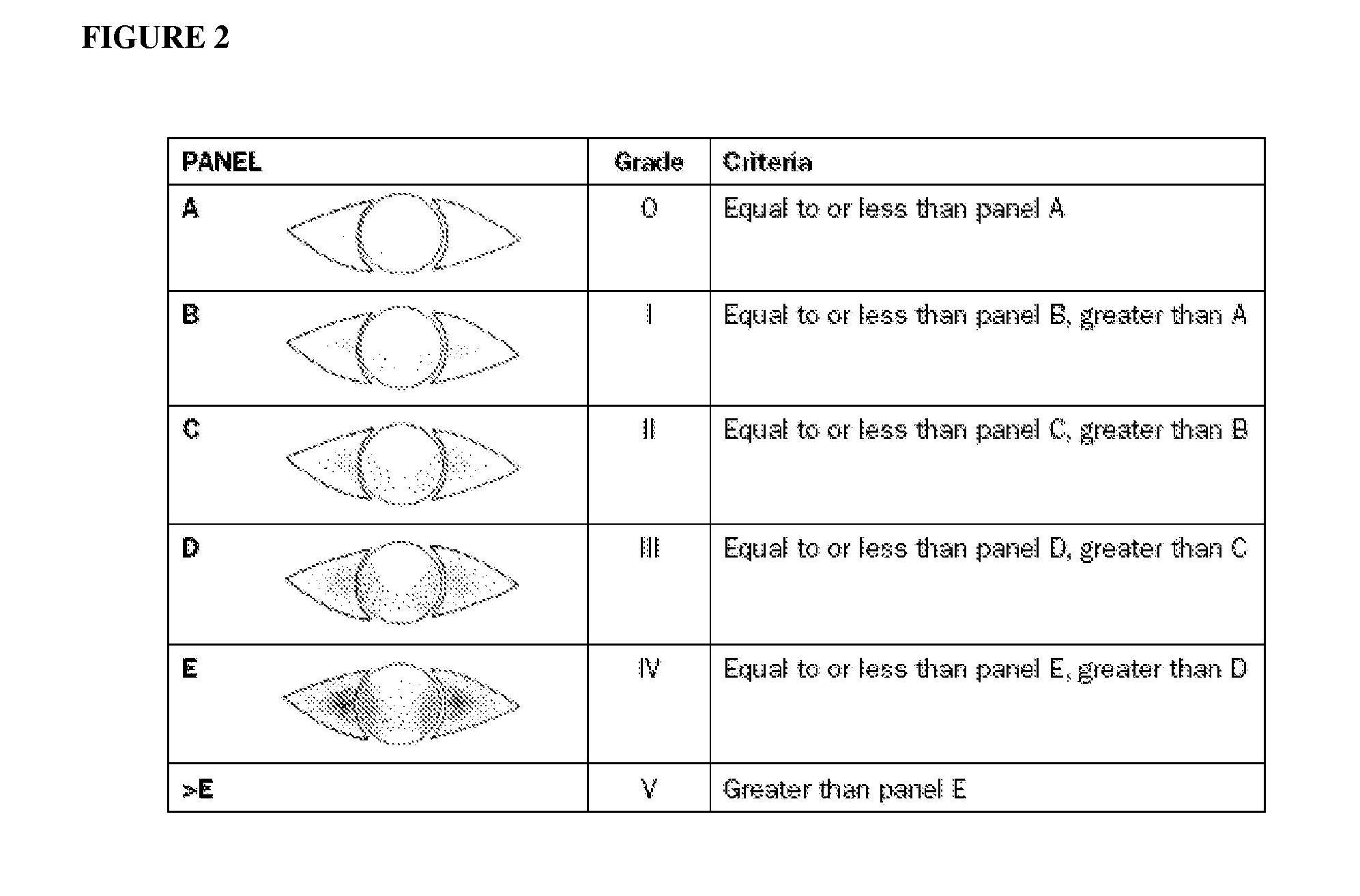
[0154]Corneal staining is a measure of epithelial disease, or break in the epithelial barrier of the ocular surface, typically seen with ocular surface disorders such as posterior blepharitis and dry eye, among others. Importantly, corneal staining can exist even without clinically evident dry eye, if there is significant lid disease, such as posterior blepharitis. Corneal staining is highly correlated with ocular discomfort in many, though not all patients; in general corneal staining is associated with high scores in the OSDI, as described above. For corneal fluorescein staining, saline-moistened fluorescein strips or 1% sodium fluorescein solution are used to stain the tear film. The entire cornea is then examined using slit-lamp evaluation with a yellow barrier filter (#12 Wratten) and cobalt blue illumination (staining is more intense when it is observed with a yellow filter). Staining is graded according to the Oxford Schema (FIG. 2).
[0155]Conj...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com