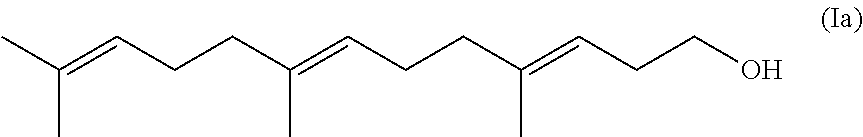
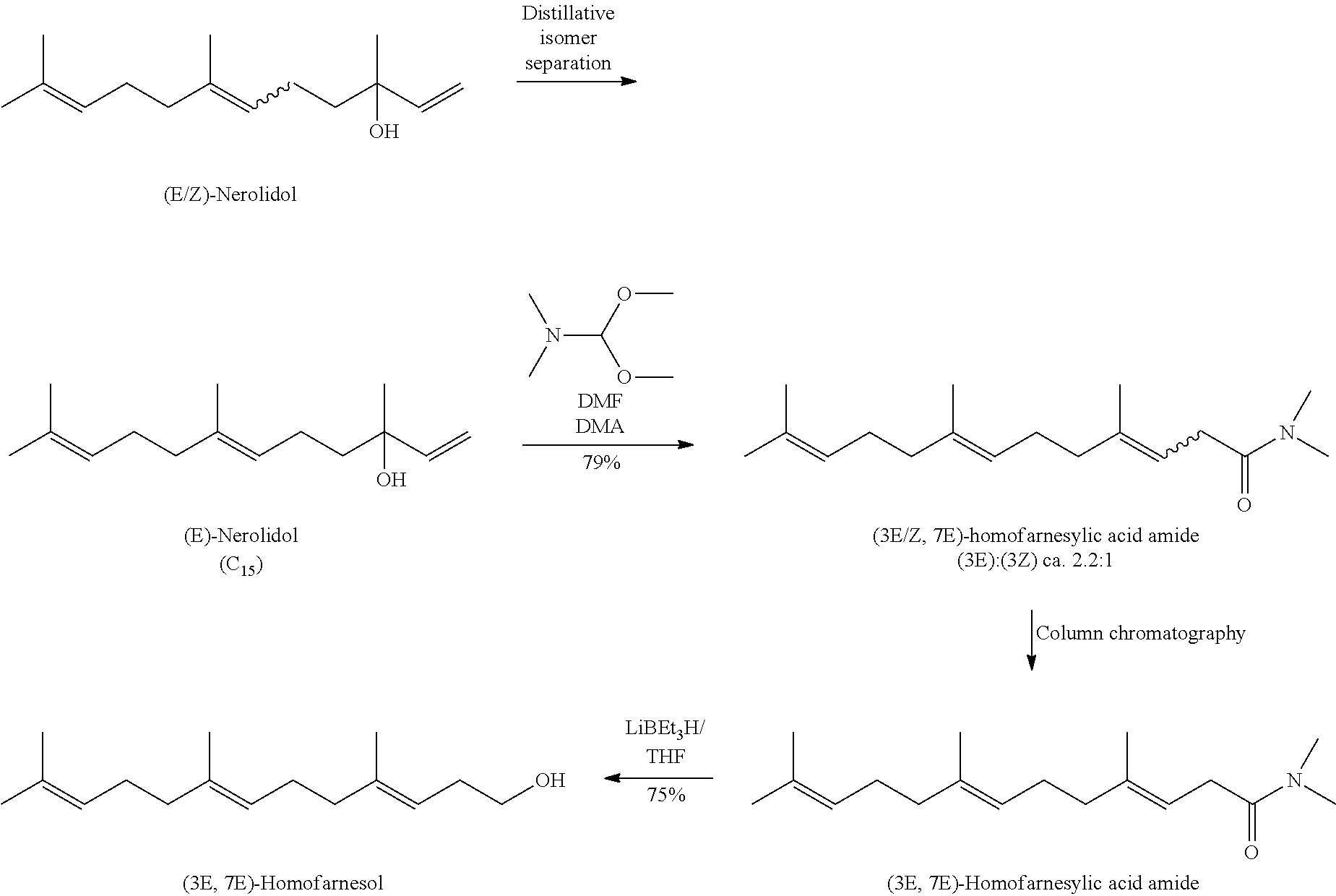
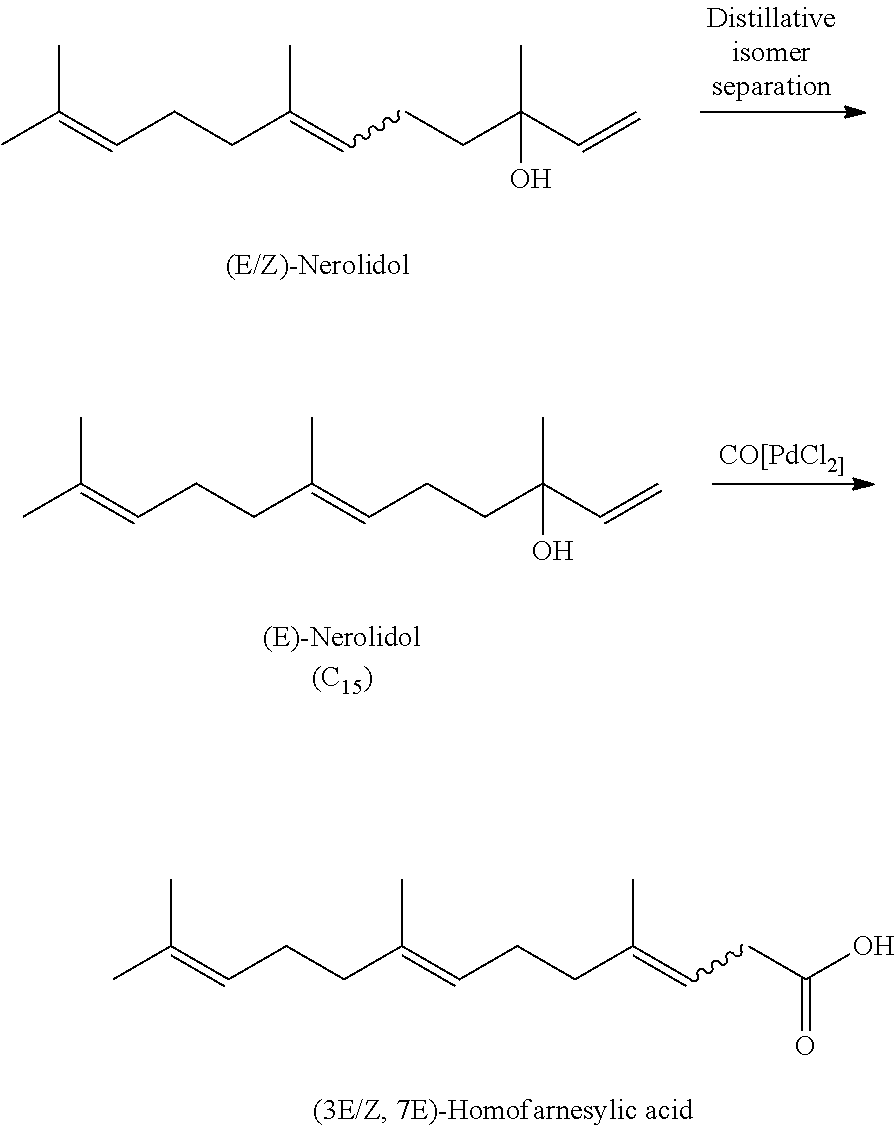
Process for the Preparation of (3E, 7E)-Homofarnesol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation examples
B) Preparation Examples
Example 1
Wittig Reaction Starting from the Cyclopropylphosphonium Salt with Potassium Tert-Butylate
[0089]
Feed Materials:
[0090]
640 ml(7890.58 mmol)ITetrahydrofuran24.7 eq 598.96 gM = 72.11 g / mol122.6 g(320 mmol)IICyclo-1 eqpropylphosphonium saltM = 383.27 g / mol35.91 g(320 mmol)IIIPotassium tert-butylate1 eqM = 112.21 g / mol48.6 g(288 mmol)IVE-Geranyl acetone0.9 eq M = 194.32 g / mol
The reactor is flushed with nitrogen. 500 ml of THF (I) are introduced as initial charge and cooled to 0° C. Cyclopropylphosphonium salt (II) comminuted in the mortar is added rinsed with the remaining amount (140 ml) of THF (I) and stirred at 0° C. for 8 min. Under an N2 atmosphere, potassium tert-butylate (III) is added, during which the internal temperature increases to 5° C. The suspension becomes immediately red-orange, and is then stirred at ca. 0° C. for 2 hours.
[0091]Geranyl acetone (IV) is added dropwise over the course of ca. 10 min (slight exothermyl), then stirring is carr...
example 2
Wittig Reaction Starting from the TPP-Bromopropane Salt with NaH
[0095]
Feed Materials:
[0096]
250 ml(3082.3 mmol)ITetrahydrofuran12.3 eq222.25 gM = 72.11 g / mol126.6 g(250 mmol)IIC4 salt HPLC - 91.67% 1 eqby weight22 g(550 mmol)IIISodium hydride in mineral 2.2 eqoil 60% strengthM = 24 g / mol48.6 g240.1 mmolIVGeranyl acetone0.96 eq90%(E) and 6%(Z)based on (E)M = 194.32 g / mol 0.9 eqand (Z)225 mmolbased on (E)
[0097]The C4 salt (II) is introduced as initial charge at room temperature in THF (I). The sodium hydride (III) is washed 3× with in each case 100 ml of n-hexane, dried in the nitrogen stream and added. The white suspension is then stirred for 4.5 h at room temperature (conversion check via HPLC). Geranyl acetone (IV) is then added and the mixture is heated at 35° C. for 21 h. 500 ml of n-heptane are then added to the yellowish suspension. THF is then removed on a rotary evaporator. The remaining suspension is admixed with 500 ml of water / methanol and the phases are separated. The aqu...
example 3
Ring Opening with BF3 Etherate and Glacial Acetic Acid
[0100]
Feed Materials:
[0101]
MolecularQuantitativeWeightAmountSubstancePurity %g / molMass gMolEqC16-90.3 (based218.38109.20.4521cyclopropaneon E & Z)Glacial acetic10060.05191431.87570.5acidBF3 etherate100141.939.050.06380.14Glacial acetic10060.052043.47.5acid
[0102]The C16-cyclopropane (I) is dissolved in glacial acetic acid (II) and produces a clear, yellow solution. The BF3 etherate is prediluted in glacial acetic acid and added over the course of 2 min at RT. The solution slowly becomes darker, no heat tonality. Stirring is carried out overnight at RT. A dark brown, clear solution (HPLC analysis) is obtained. After 27 h at RT, 2.5 l of water and 1 l of cyclohexane are added to the clear, dark brown solution, and the phases are separated. The aqueous phase is extracted twice with 0.5 l cyclohexane. The combined organic phases are washed in succession with 4×0.2 l of water, 0.25 l of saturated NaHCO3 solution and again 0.25 l of wat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com