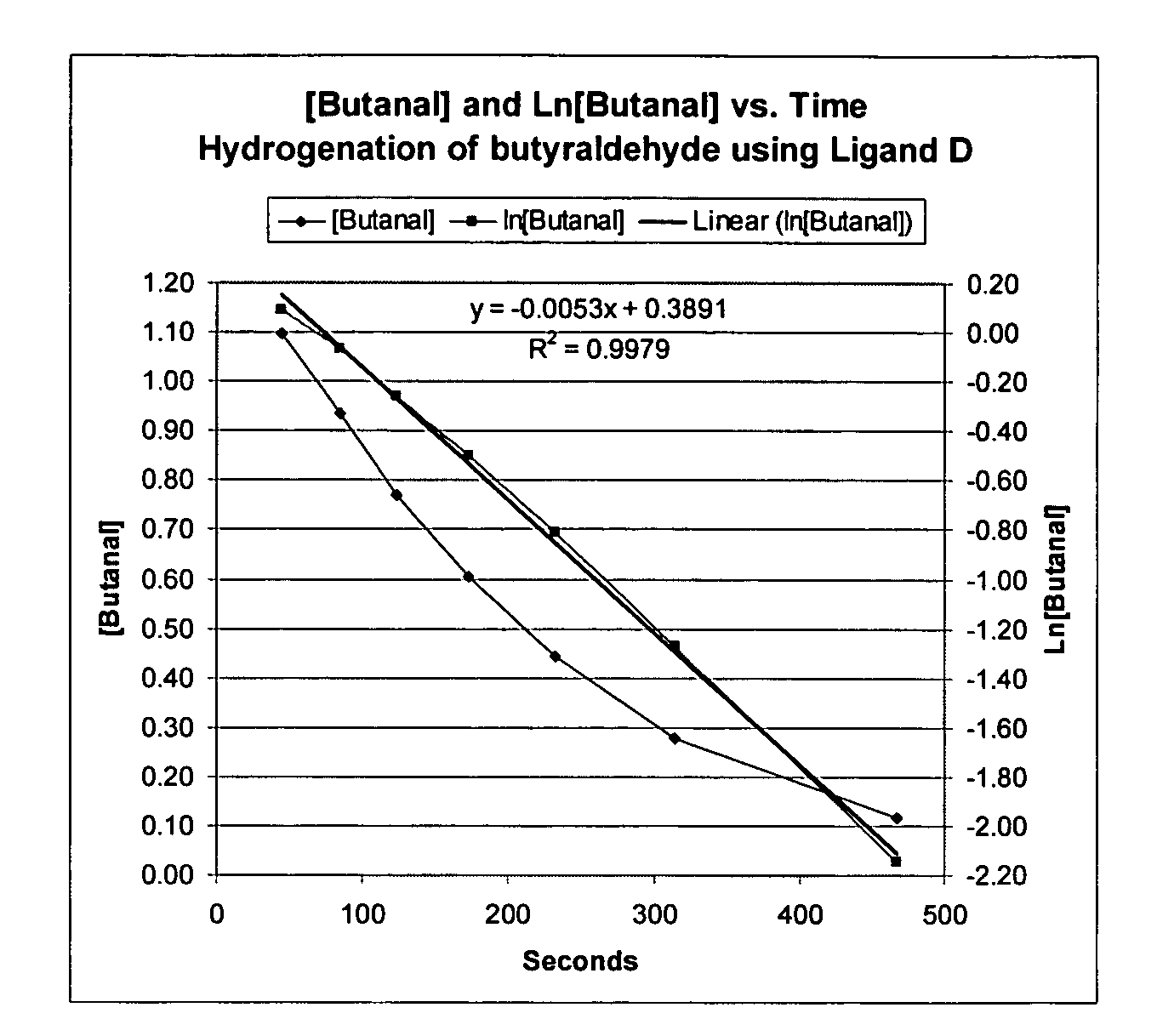
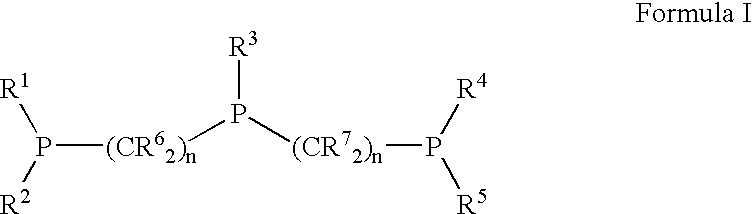
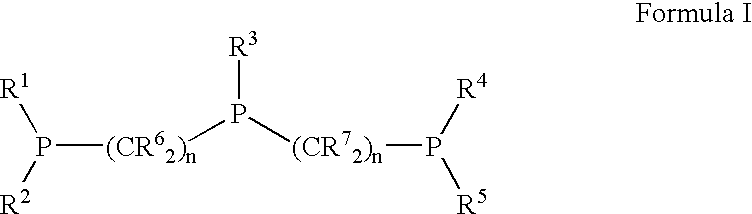
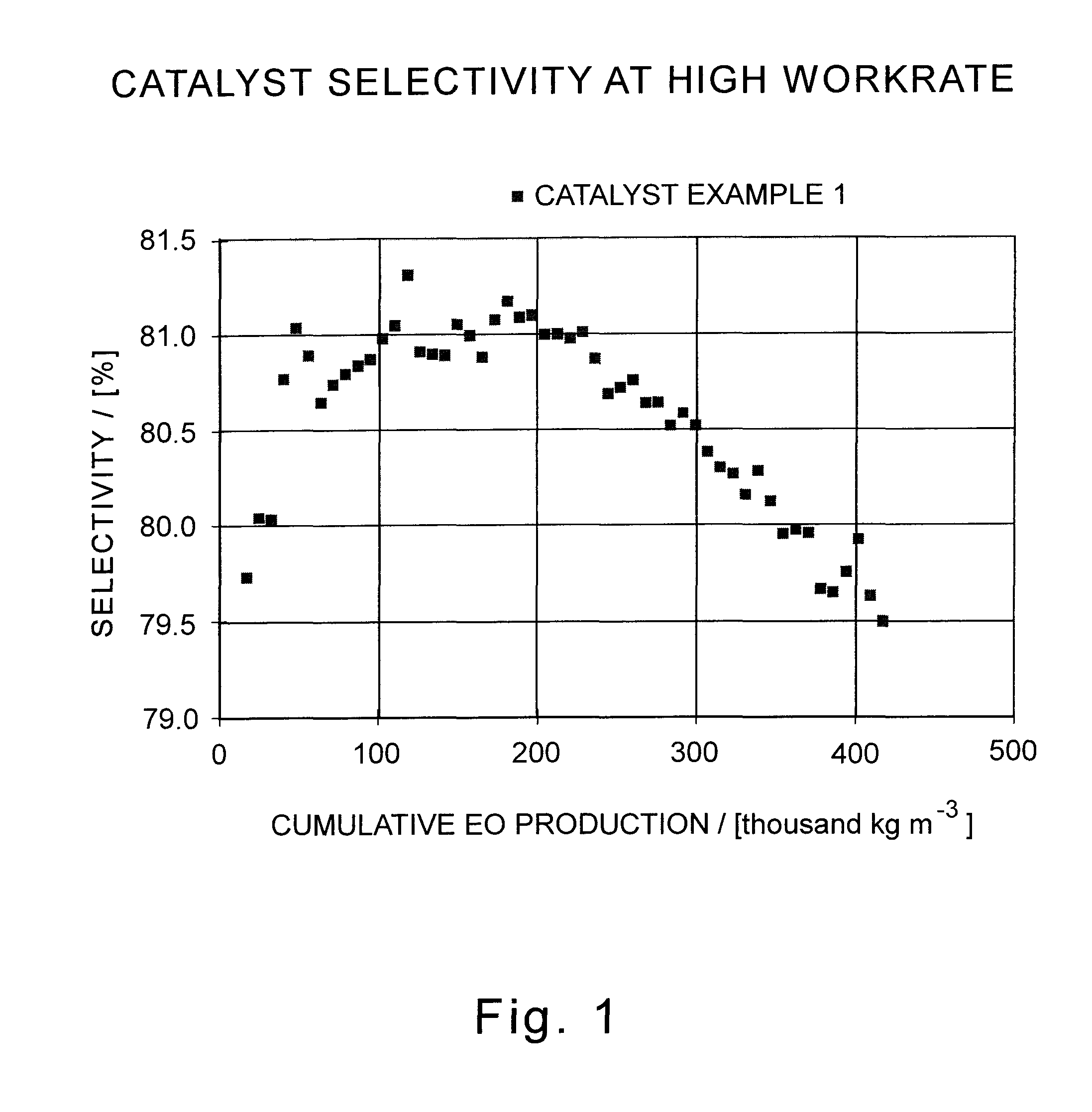
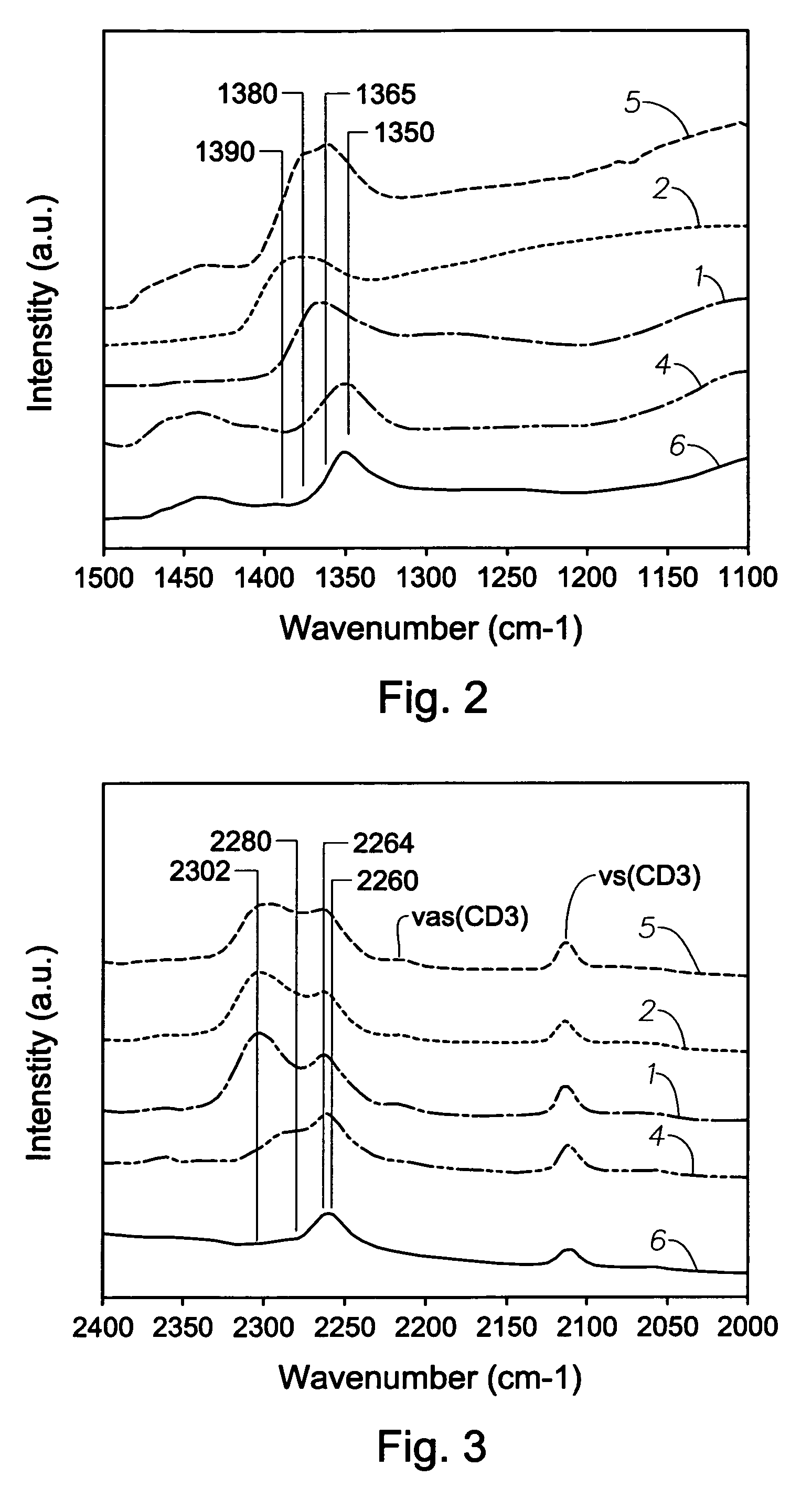
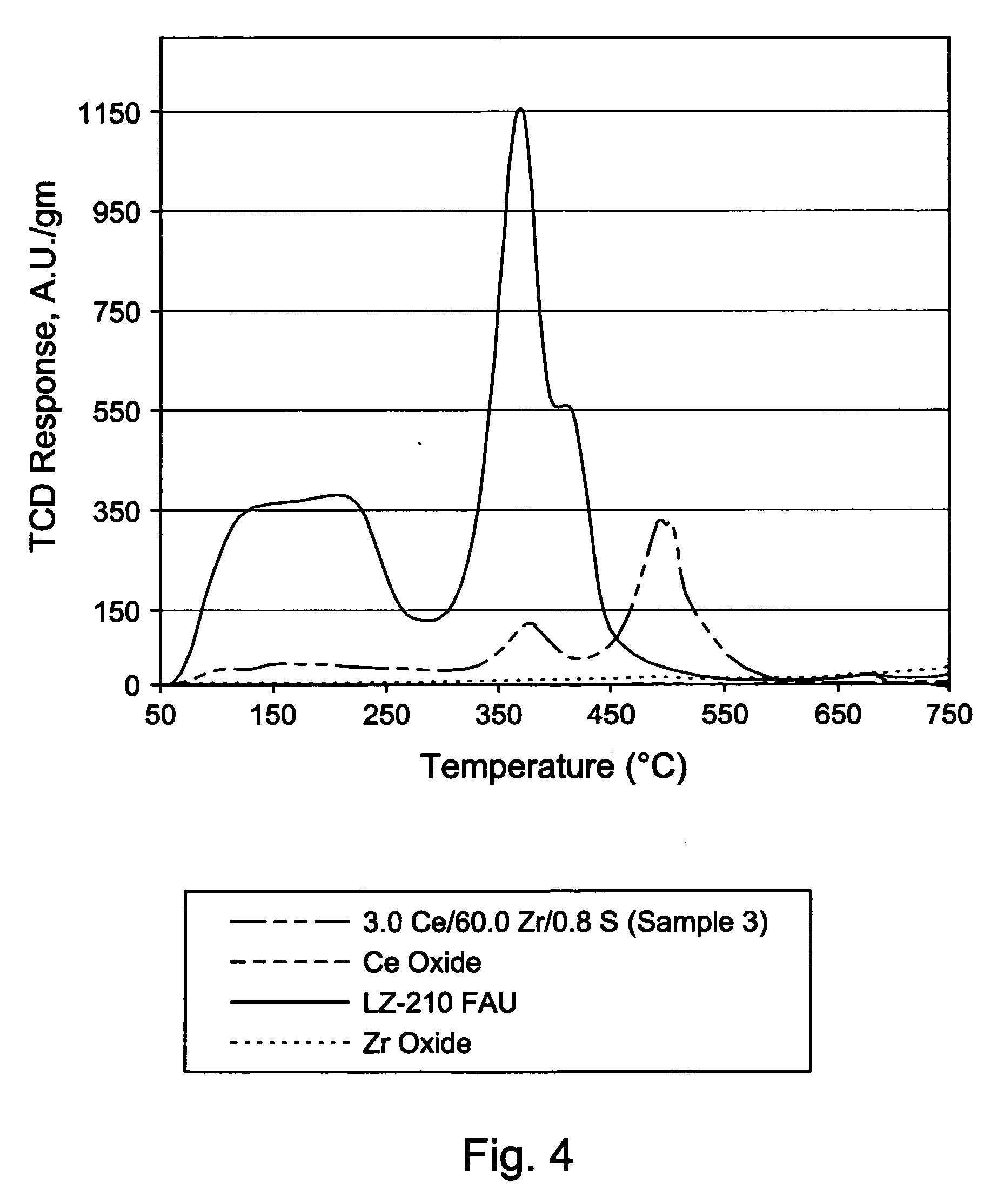
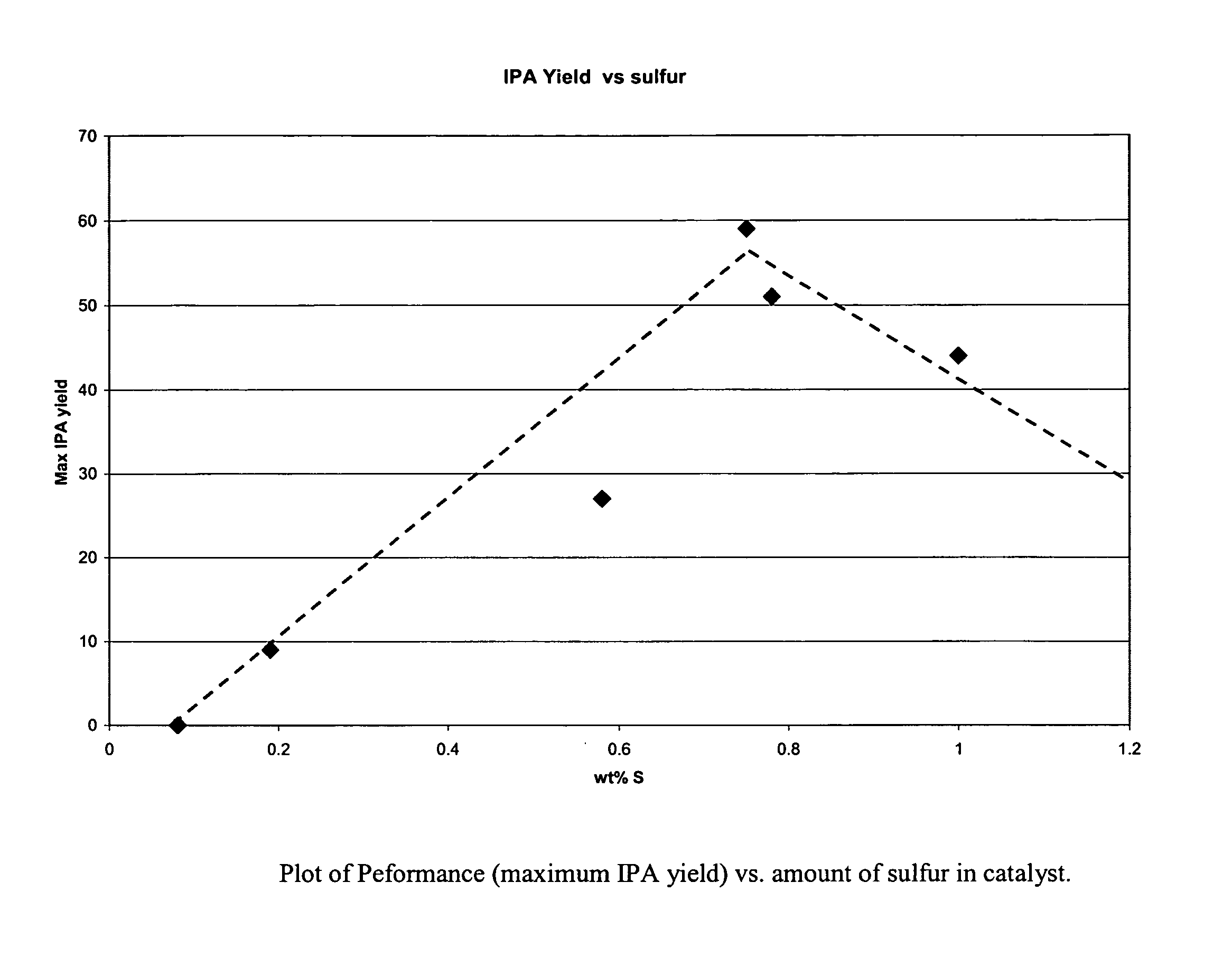
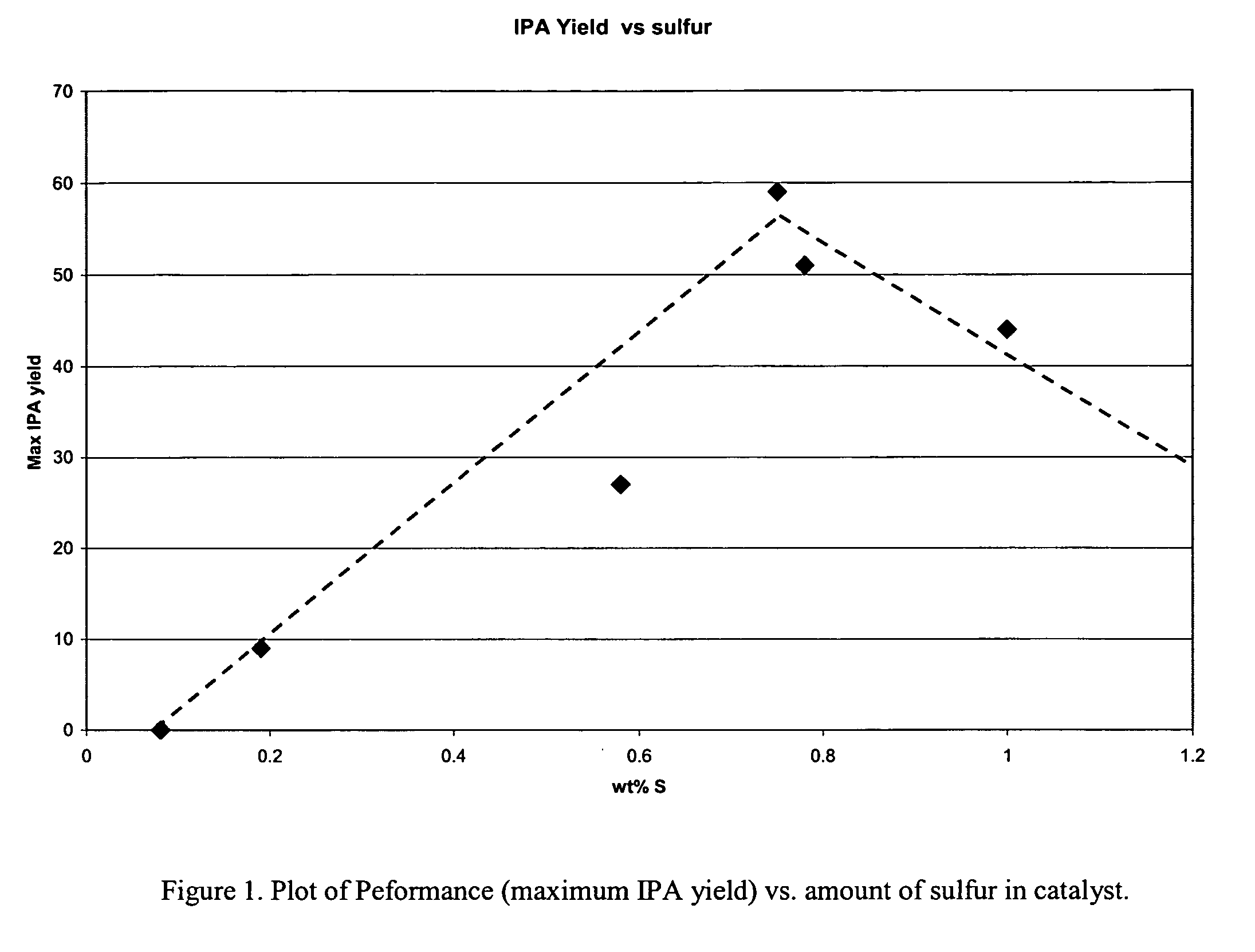
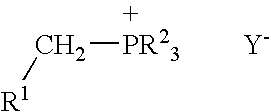Patents
Literature
50results about "Oxygen compounds preparation by alkyne addition" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oxide catalyst composition
InactiveUS7012039B2High selectivityLow selectivityOrganic compound preparationCarboxylic acid esters preparationLanthanideMethacrolein
An oxide catalyst composition for use in producing methacrolein or a mixture of methacrolein and methacrylic acid, wherein the oxide catalyst composition is represented by the formula (Mo+W)l2BiaAbBcFedXeSbfOg, wherein: A is at least one member selected from the group consisting of Y and the elements of the lanthanoid series exclusive of Pm; B is at least one member selected from the group consisting of K, Rb and Cs; X is Co solely, or a mixture of Co and at least one member selected from the group consisting of Mg and Ni; and a, b, c, d, e, f and g are, respectively, the atomic ratios of Bi, A, B, Fe, X, Sb and O, relative to twelve atoms of the total of Mo and W, wherein the atomic ratios (a to f) of the elements and the relationship between the amounts of the elements are chosen so as to satisfy specific requirements.
Owner:ASAHI KASEI CHEM CORP
Hydroformylation process
InactiveUS6049011AAvoid the needReduce processingPreparation by oxo-reaction and reductionPreparation by hydrogenationEthyleneHydrocarbon
PCT No. PCT / EP96 / 00163 Sec. 371 Date Oct. 15, 1997 Sec. 102(e) Date Oct. 15, 1997 PCT Filed Jan. 17, 1996 PCT Pub. No. WO96 / 22265 PCT Pub. Date Jul. 25, 1996A dilute ethylene stream, e.g., one produced by steam cracking, is oxonated to yield propanal, without the need to separate other lower hydrocarbons.
Owner:EXXON CHEM PAT INC
Process for preparing functional group-containing olefinic compounds
ActiveUS6838576B1High yieldStereoselectivity can be controlledPreparation by ester-hydroxy reactionOrganic compound preparationCompound aLeaving group
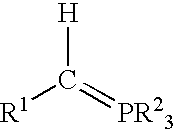
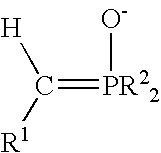
A process for preparing functional group-containing olefinic compounds comprises the steps of (a) reacting at least one alkylidene phosphorane with at least one carbonyl-containing compound that comprises at least one group that is a leaving group, or that is capable of subsequent conversion to a leaving group, to form an olefinic compound that comprises at least one leaving group, the carbonyl-containing compound being selected from the group consisting of ketones and aldehydes; and (b) reacting the olefinic compound with at least one functional group-containing nucleophile to form a functional group-containing olefinic compound.
Owner:3M INNOVATIVE PROPERTIES CO
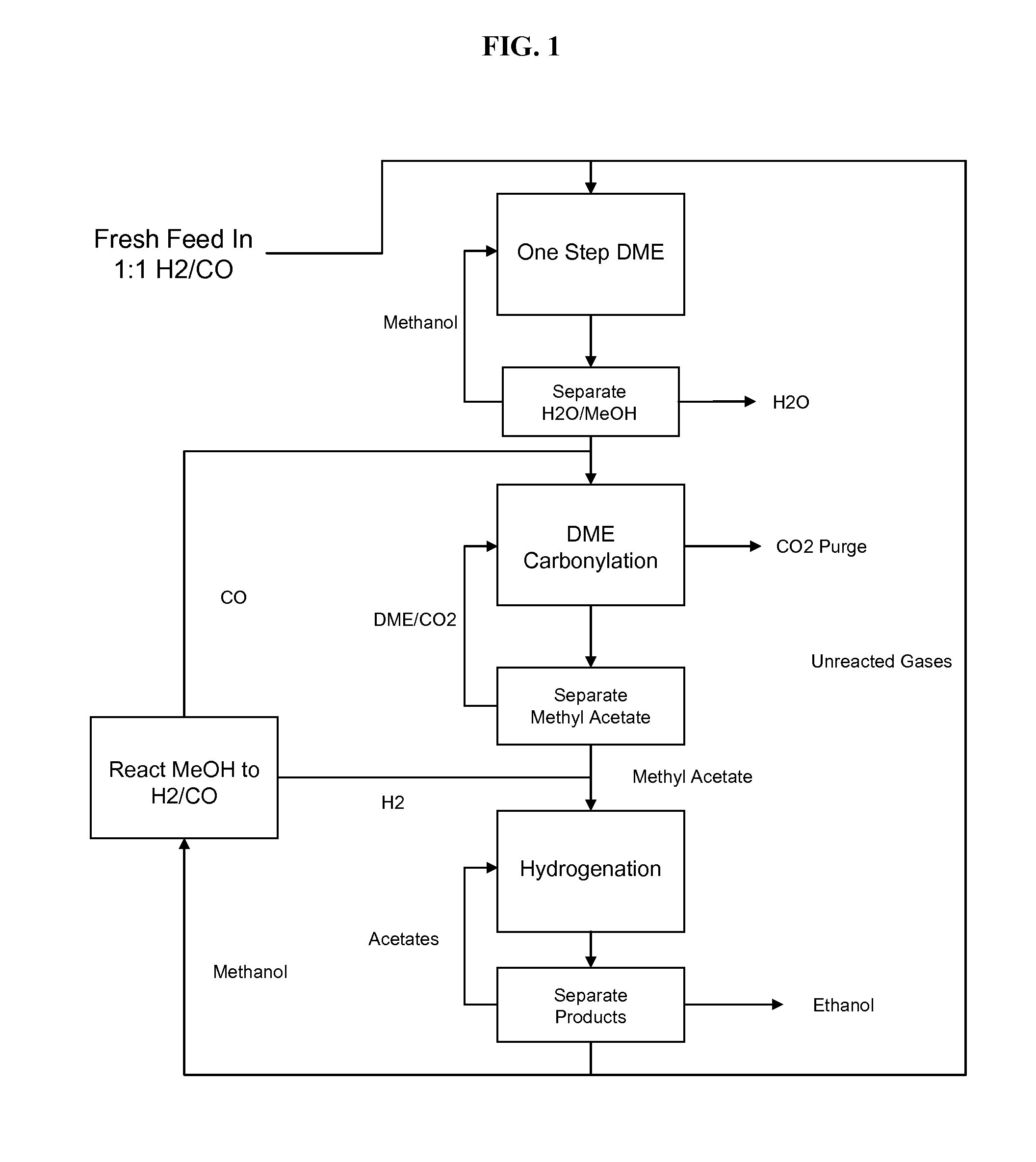
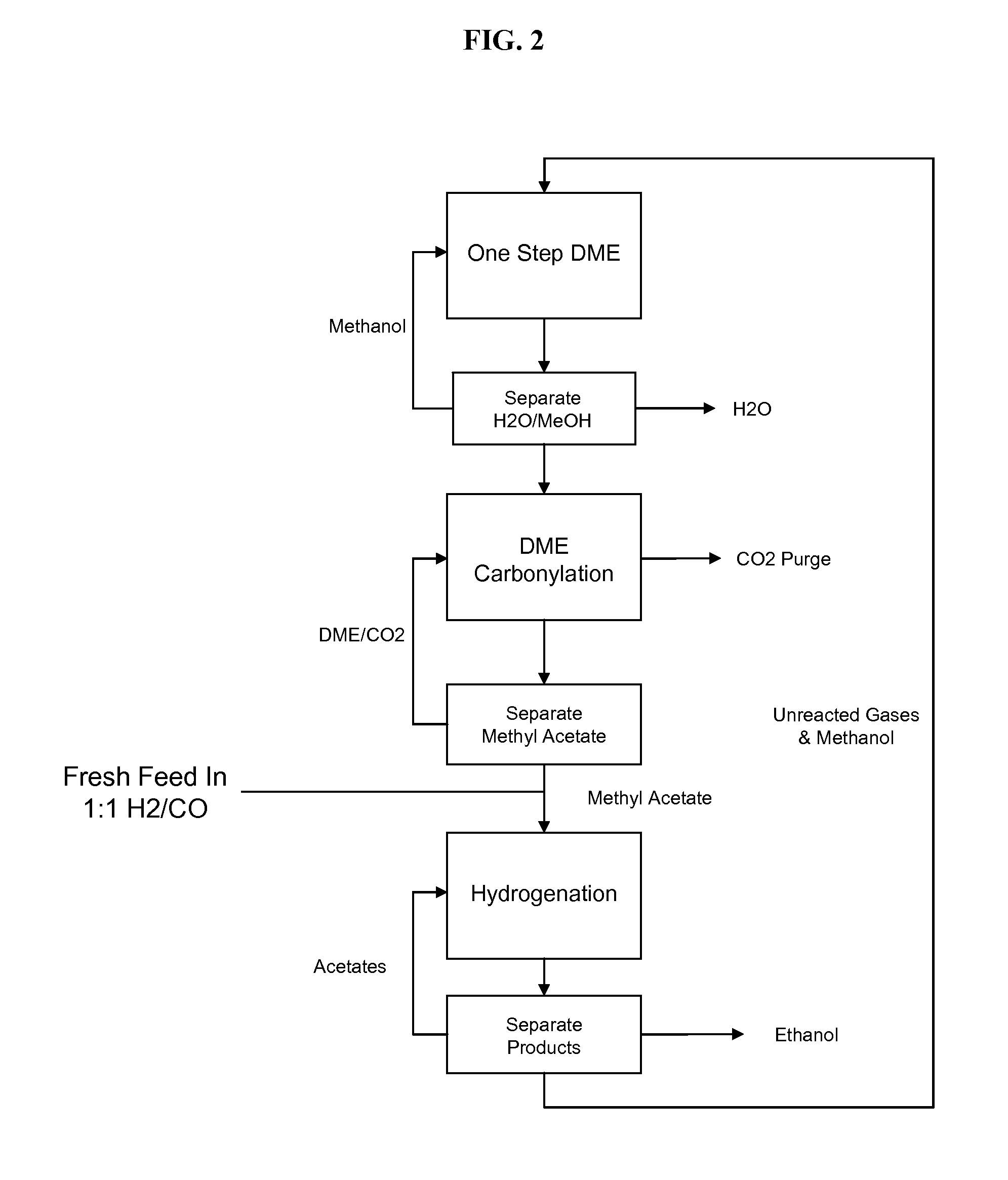
Selective process for conversion of syngas to ethanol
ActiveUS20110124927A1Organic compound preparationOxygen compounds preparation by reductionSyngasUnit operation
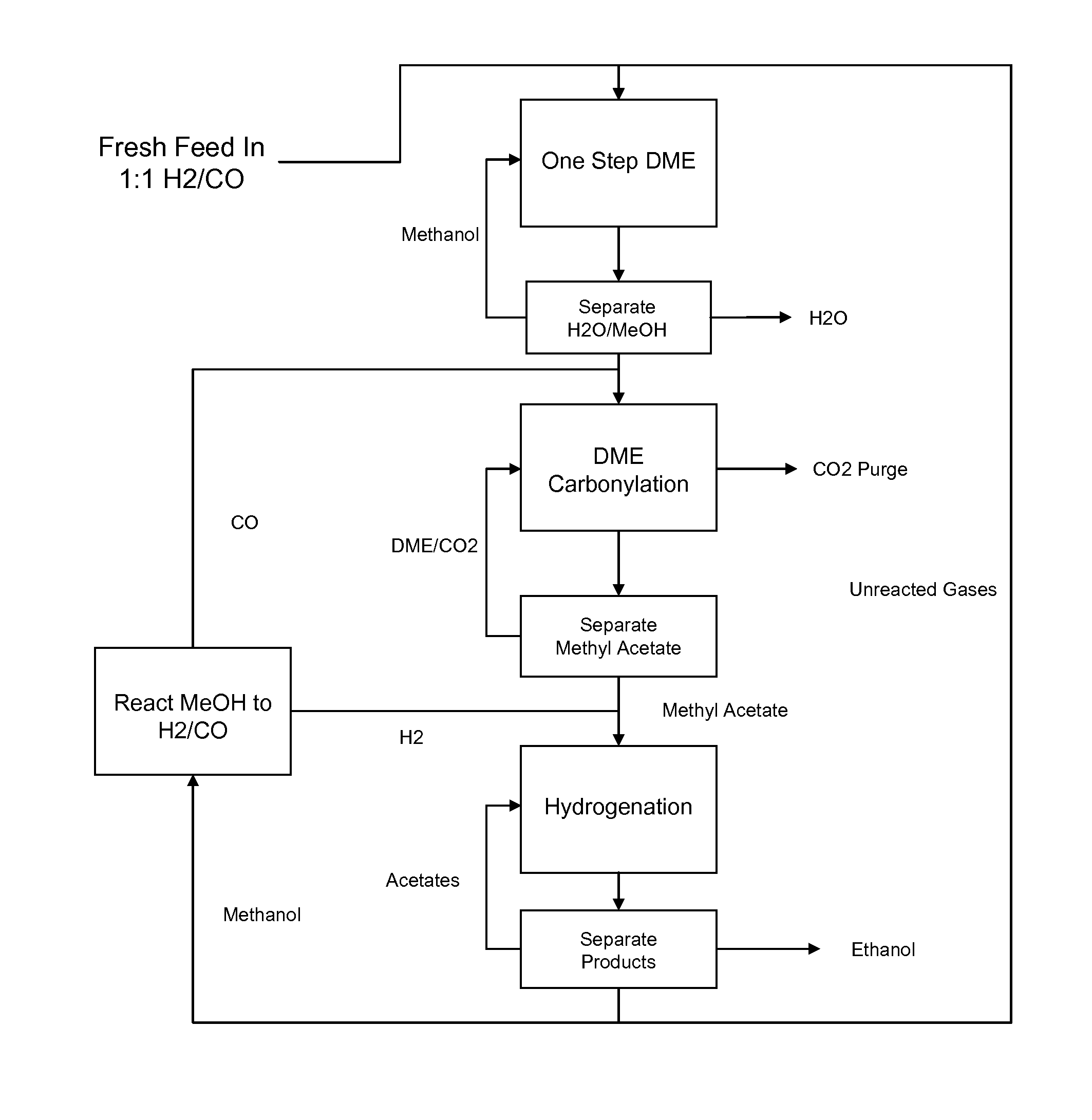
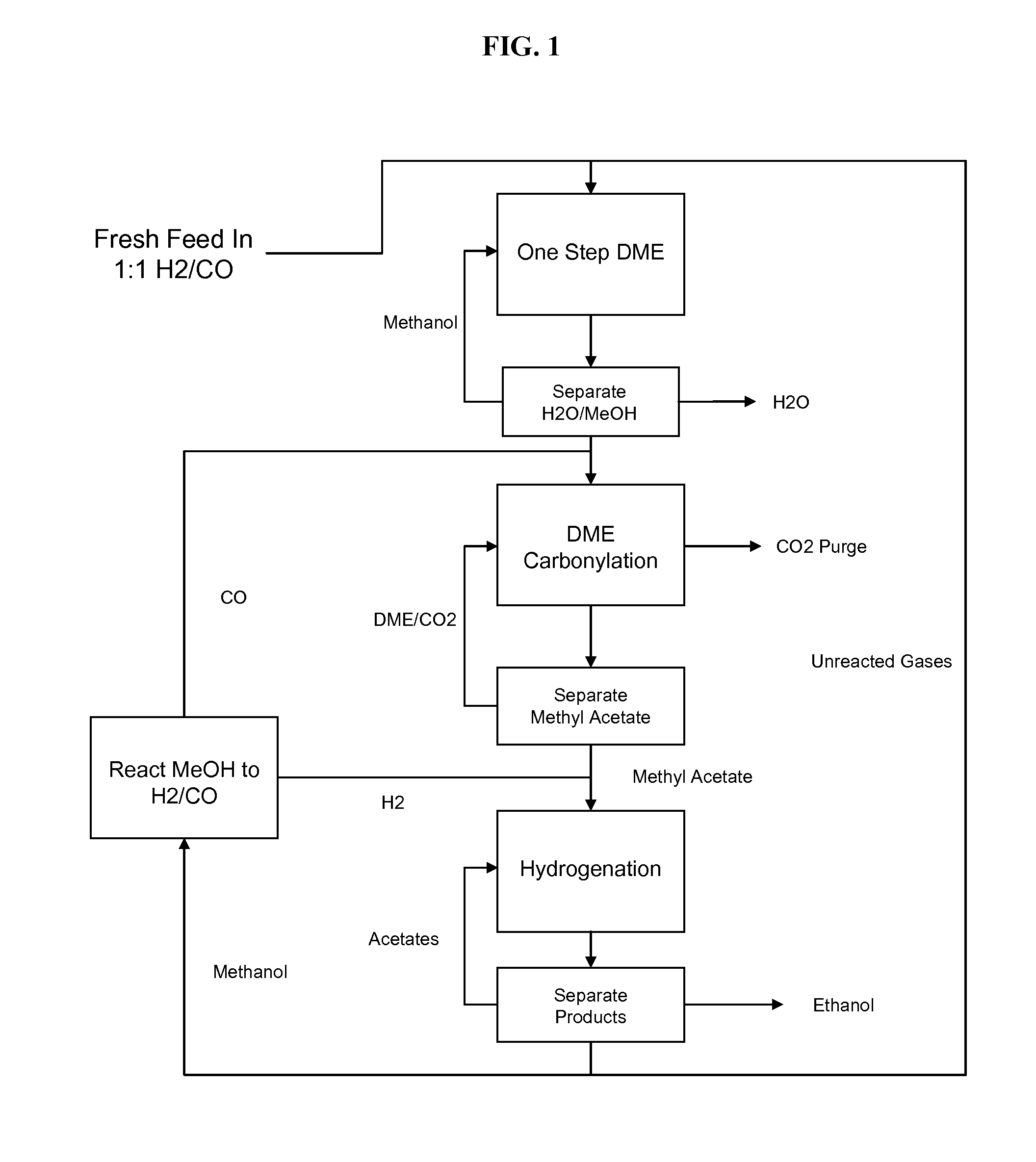
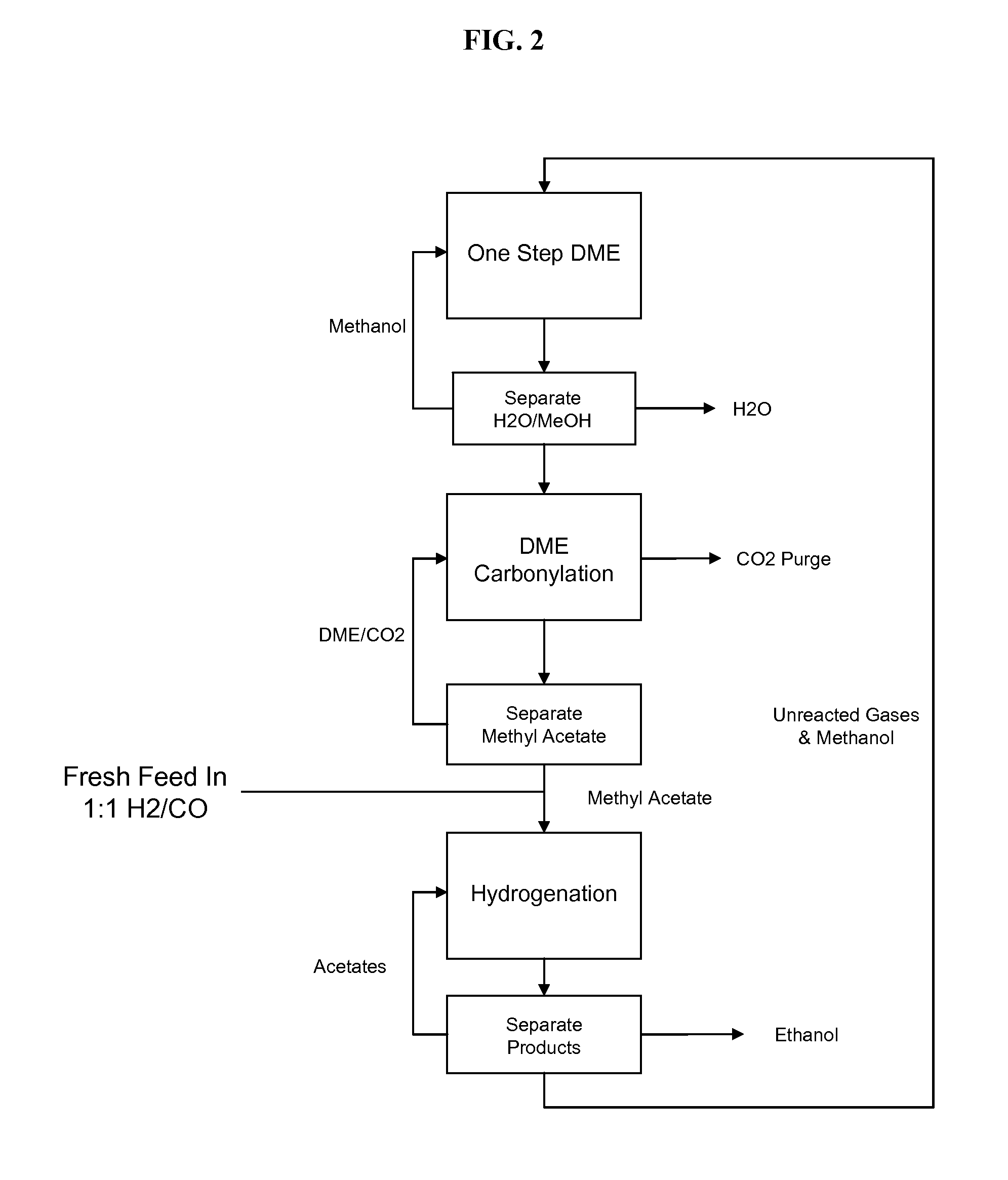
The present invention provides processes for selectively producing ethanol from syngas. In some variations, the process comprises converting biomass-derived syngas to dimethyl ether, carbonylating the dimethyl ether to methyl acetate, hydrogenating the methyl acetate to methanol and ethanol, and recovering the ethanol product. The methanol is preferably recycled by converting to hydrogen and carbon monoxide for introduction back into the process at distinct points. In certain variations of this invention, fresh syngas feed is introduced downstream of the first unit operation in the sequence. High yields of ethanol from biomass can be achieved according to the disclosed processes.
Owner:ALBEMARLE CORP
Process for preparing a catalyst, the catalyst, and a use of the catalyst
InactiveUS20050222442A1Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsDopantPolymer science
Owner:SHELL OIL CO
Hydrogenation of acetone
ActiveUS7041857B1Promote conversionSpeed up reactionOrganic compound preparationBeer fermentationHydrogenMetal catalyst
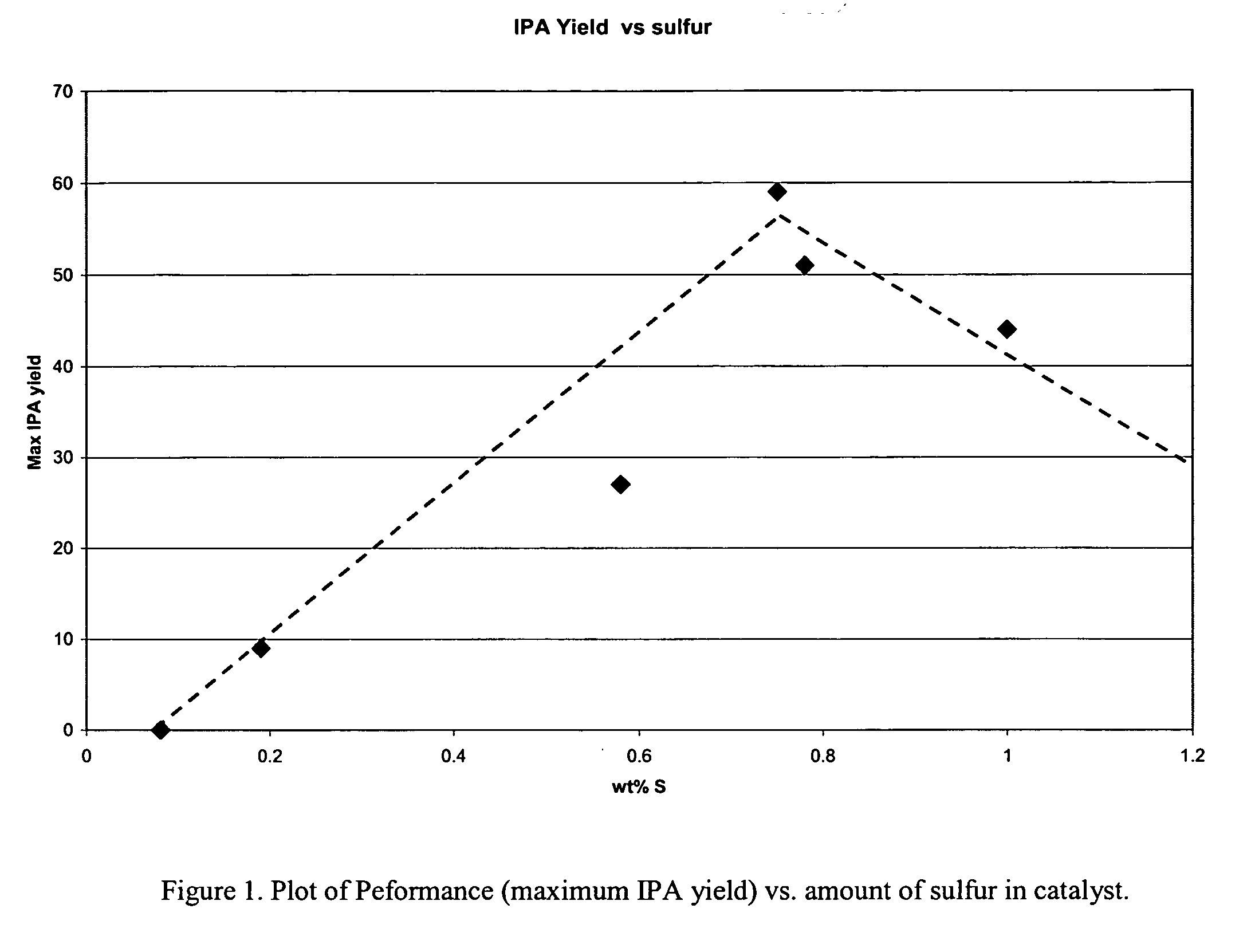
This invention relates to an improvement in a liquid phase process for producing isopropanol by the hydrogenation of acetone in the presence of a hydrogenation catalyst; the improvement comprising;contacting acetone with hydrogen under continuous liquid phase conditions; and,employing a sponge metal catalyst promoted with an effective amount of chromium.
Owner:TAMINCO NV
Process for hydrogenation of carbonyl and iminocarbonyl compounds using ruthenium catalysts comprising tetradentate diimino-diphosphine ligands
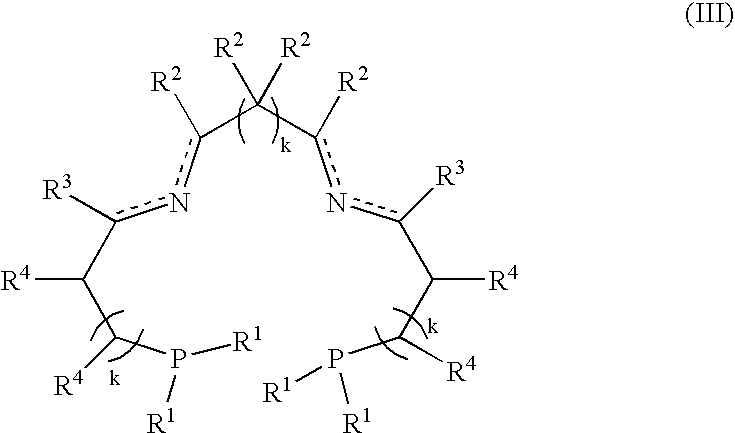
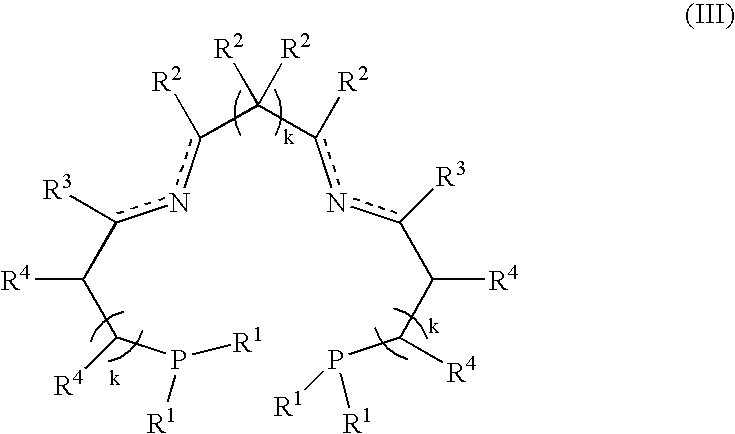
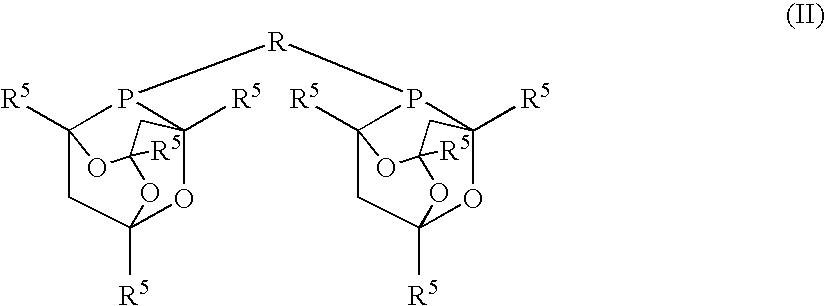
A process for the hydrogenation, using molecular hydrogen (H2), of a catalytic system, wherein the catalytic system comprises a base and a complex of formula; (II) wherein Y represents simultaneously or independently a hydrogen or halogen atom, a hydroxy group, or an alkoxy, carboxyl or other anionic radical, and N2P2 is a tetradentate diimine-diphosphino ligand.
Owner:FIRMENICH SA
Process for hydrogenation of carbonyl and iminocarbonyl compounds using ruthenium catalysts comprising tetradentate diimino-diphosphine ligands
A process for the hydrogenation, using molecular hydrogen (H2) of a catalytic system, wherein the catalytic system includes a base and a complex of formula (II):Ru(P2N2)Y2 (II)wherein Y represent simultaneously or independently a hydrogen or halogen atom, a hydroxy group, or an alkoxy, carboxyl or other anionic radical, and P2N2 is a tetradentate diimino-diphosphine ligand.
Owner:FIRMENICH SA
Method for producing guerbet alcohols
The invention relates to a method for producing Guerbet alcohols of formula (I), CH3(CH2)nCHR<1>CH2OH, wherein R<1 >represents a linear alkyl radical having n-1 carbon atoms, and n represents 3 to 9 carbon atoms. Fatty alcohols of formula (II), R<2>OH, wherein R<2 >represents linear alkyl radicals having 6 to 12 carbon atoms, are condensed in the presence of carbonyl compounds and alkali hydroxides.
Owner:COGNIS DEUT GMBH & CO KG
Process for hydrolyzing di-isopropyl ether to isopropyl alcohol by catalytic distillation using a solid acid catalyst
InactiveUS6906229B1High yieldLong catalyst lifeOrganic compound preparationChemical industrySolid acidCatalytic distillation
Process for Producing Ethanol by Hydrocarbon Oxidation and Hydrogenation or Hydration
InactiveUS20130261347A1Preparation by oxo-reaction and reductionOrganic compound preparationAcetic acidPhotochemistry
Hydrocarbons are oxidized to ethylene and / or oxygenates that comprise acetic acid. The acetic acid may be converted to ethanol by hydrogenation. The ethylene may be converted to ethanol by hydration.
Owner:CELANESE INT CORP
Novel tridentate phosphines and method of forming aldehyde hydrogenation catalysts
ActiveUS20060116536A1Preparation by oxo-reaction and reductionGroup 5/15 element organic compoundsHydrogenAlcohol
This invention comprises a process for hydrogenation of aldehydes to alcohols using novel homogeneous catalysts. The catalysts are generated in situ under hydrogen and carbon monoxide gases in a suitable solvent, by mixing a rhodium catalyst precursor, such as Rh(CO)2 acetoacetonate and a defined ligand.
Owner:DOW GLOBAL TECH LLC
Alkylene oxide catalyst and use thereof
ActiveUS20140088316A1Selectivity towards ethylene oxide formation is optimalGood choiceOrganic compound preparationCatalyst activation/preparationEthylene DibromideOxygen
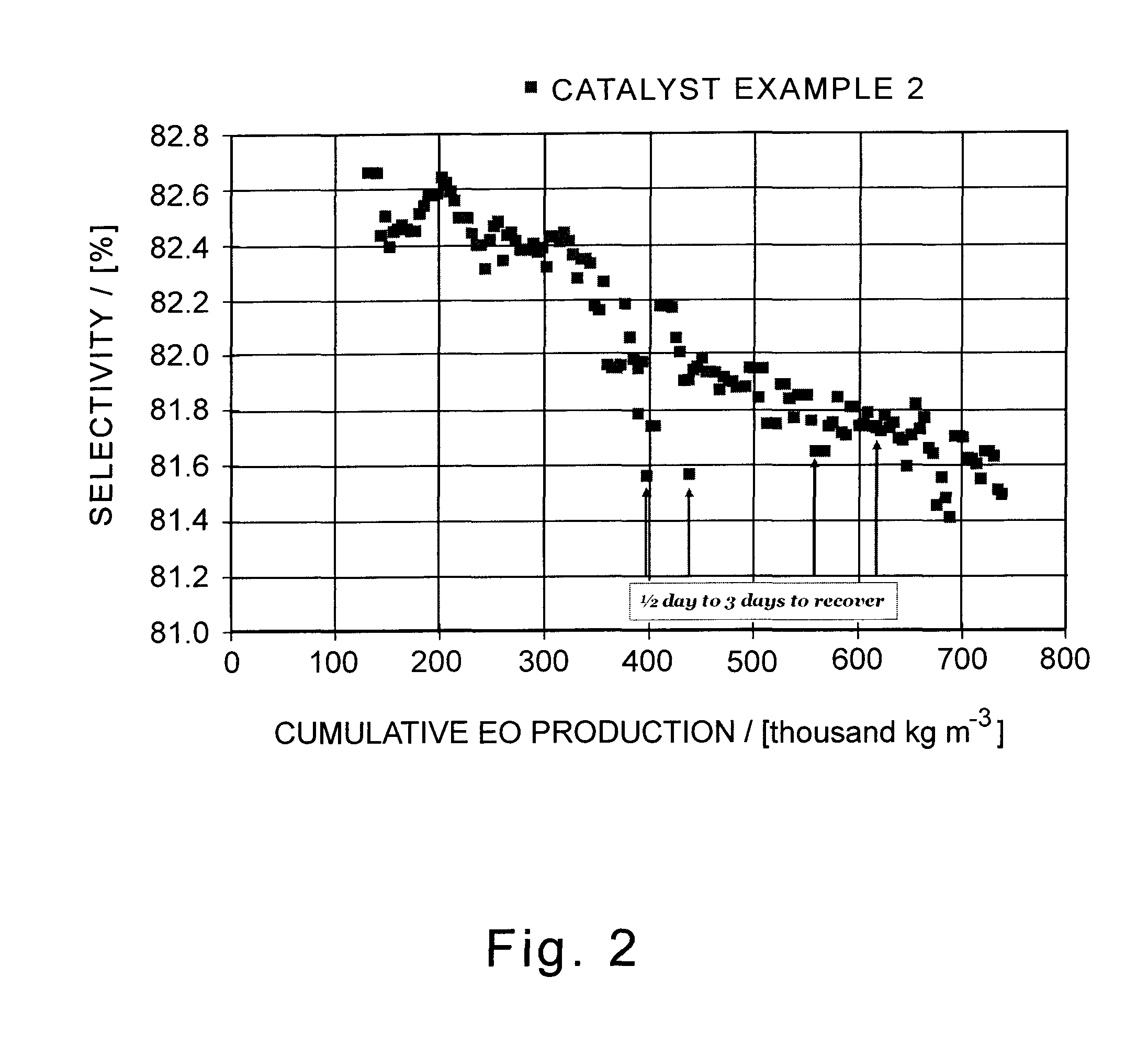
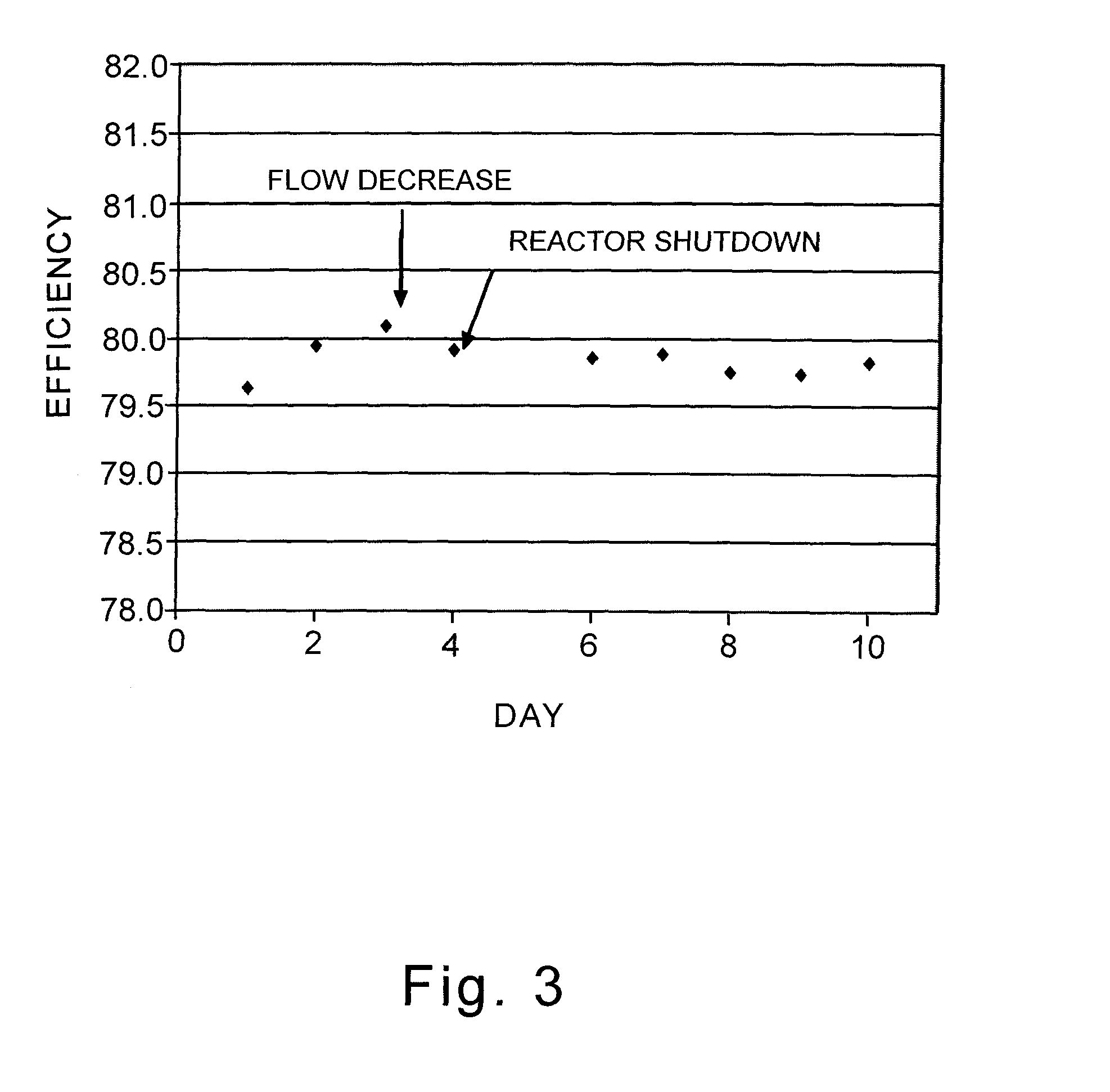
A supported silver catalyst and use thereof in a process for producing an alkylene oxide, such as ethylene oxide, by the direct oxidation of an alkylene with oxygen or an oxygen-containing gas, wherein the catalyst provides improved stability and improved resilience to reactor upsets and timely recovery to substantially pre-upset levels of catalyst activity and / or efficiency. In some embodiments, the catalyst also exhibits improved activity. A catalyst capable of producing ethylene oxide at a selectivity of at least 87 percent while achieving a work rate of at least 184 kg / h / m3 at a temperature of no greater than 235° C. when operated in a process where the inlet feed to a reactor containing the catalyst comprises ethylene, oxygen, and carbon dioxide, wherein the concentration of carbon dioxide in the inlet feed is greater than or equal to 2 mole percent.
Owner:DOW GLOBAL TECH LLC
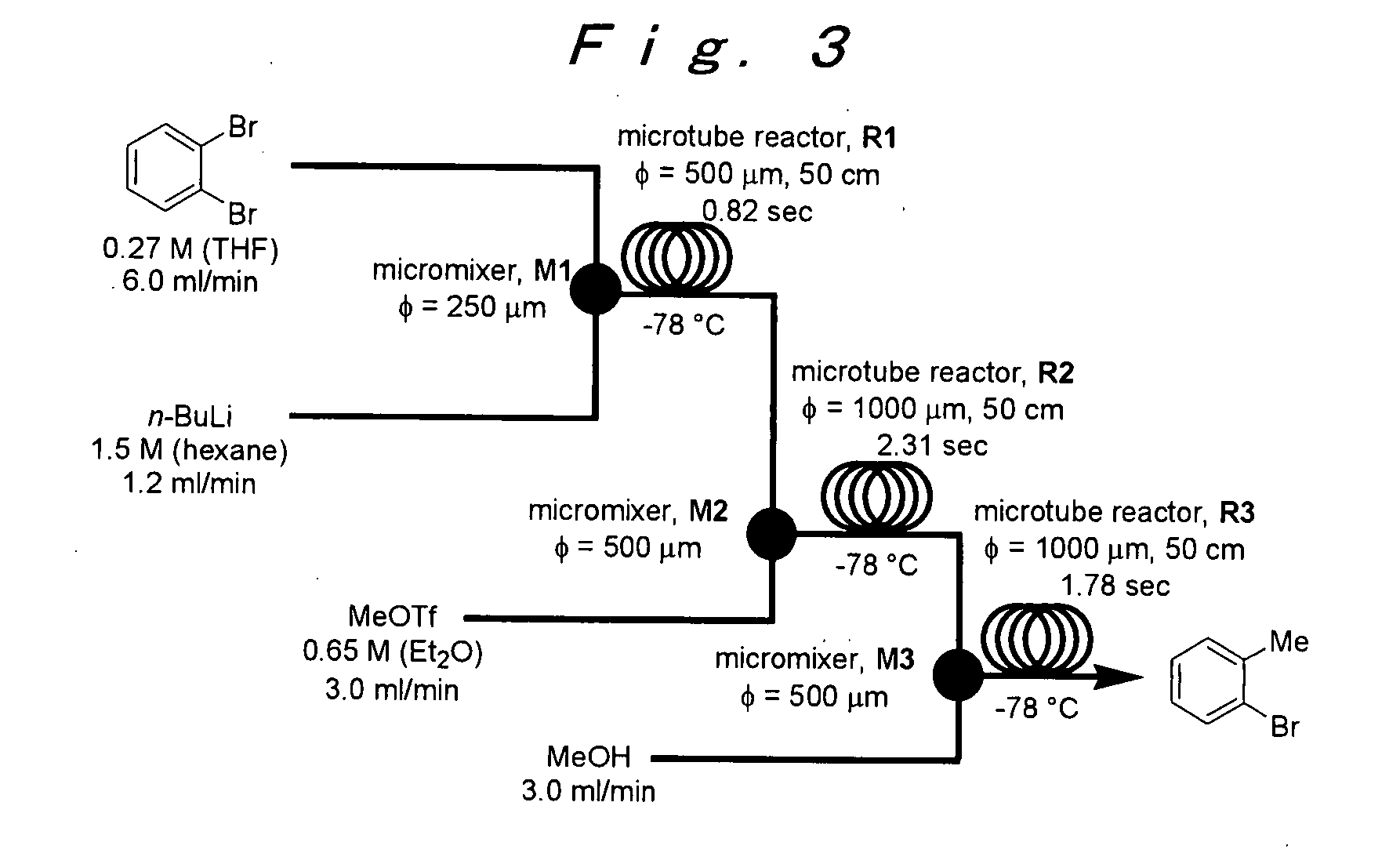
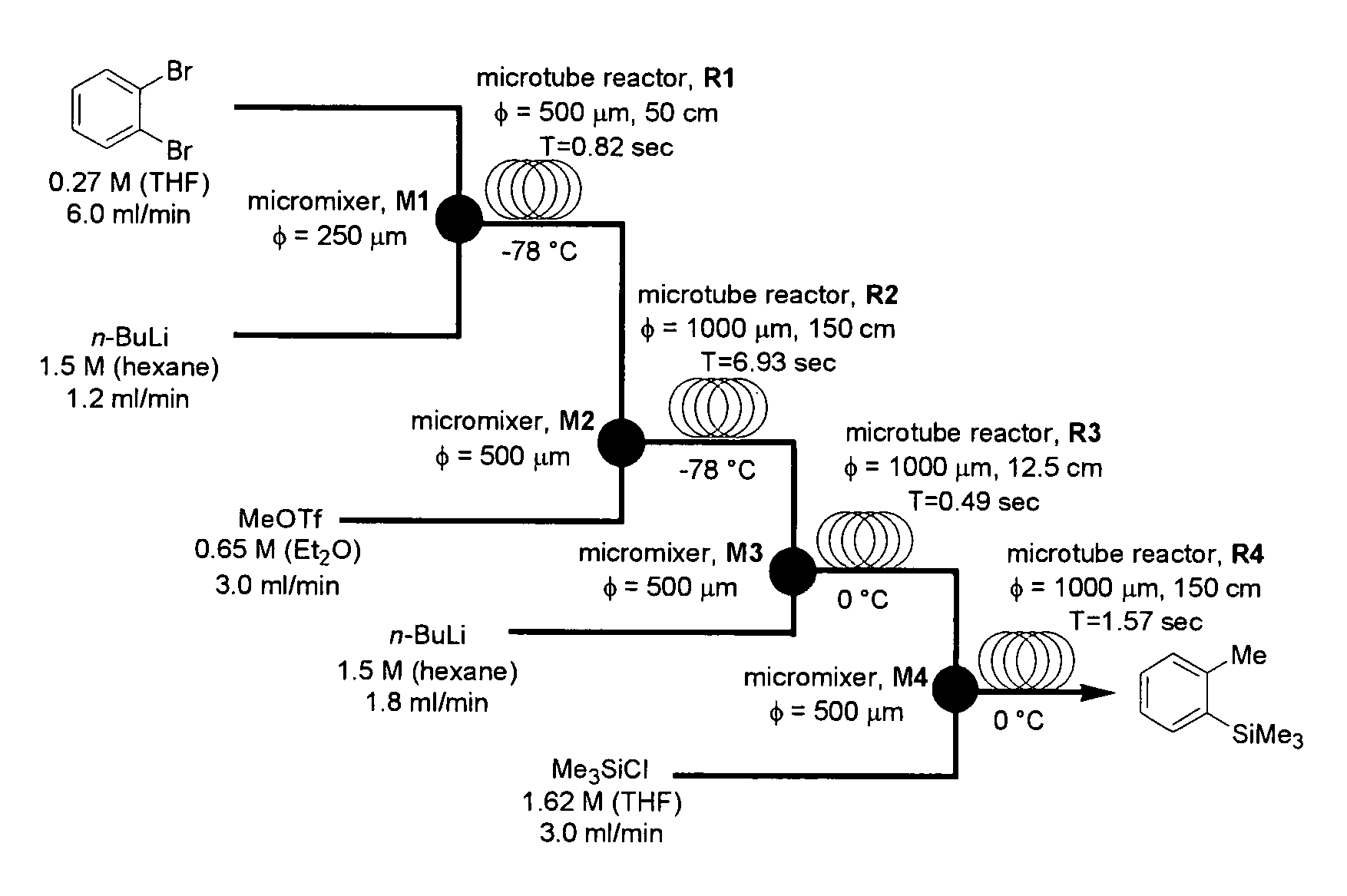
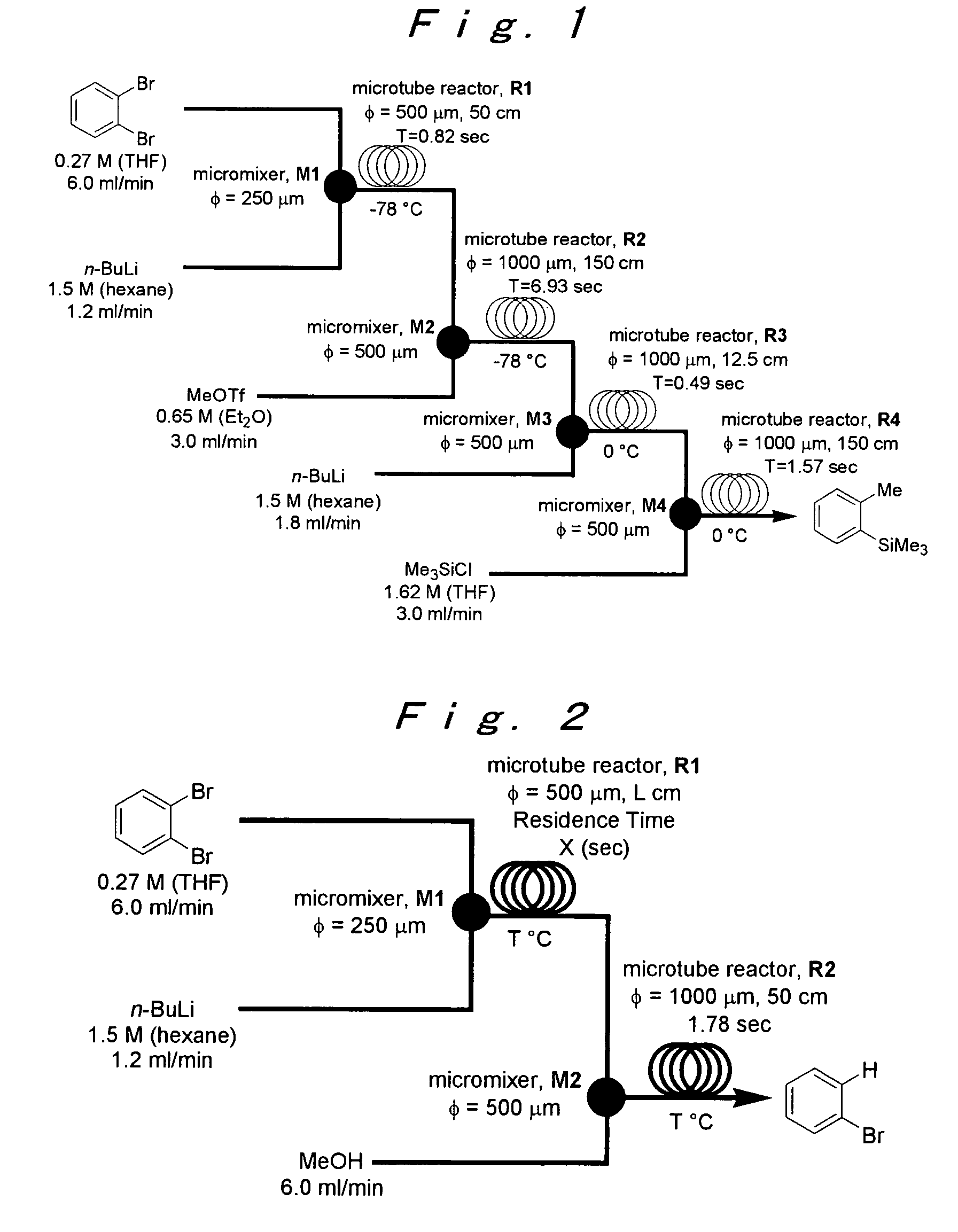
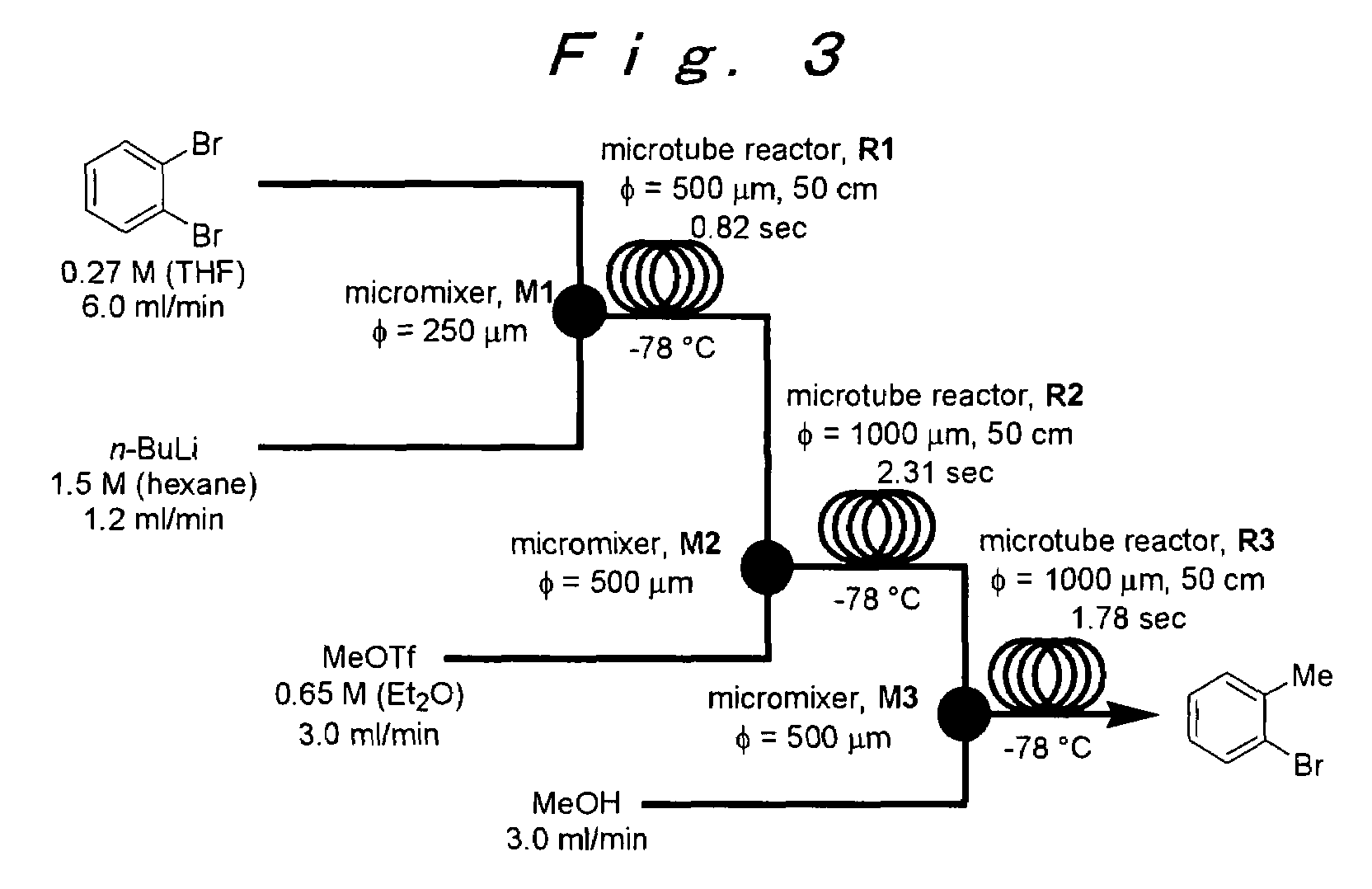
Method of producing an o-disubstituted aromatic compound, and method of producing a monosubstituted-monohaloaromatic compound
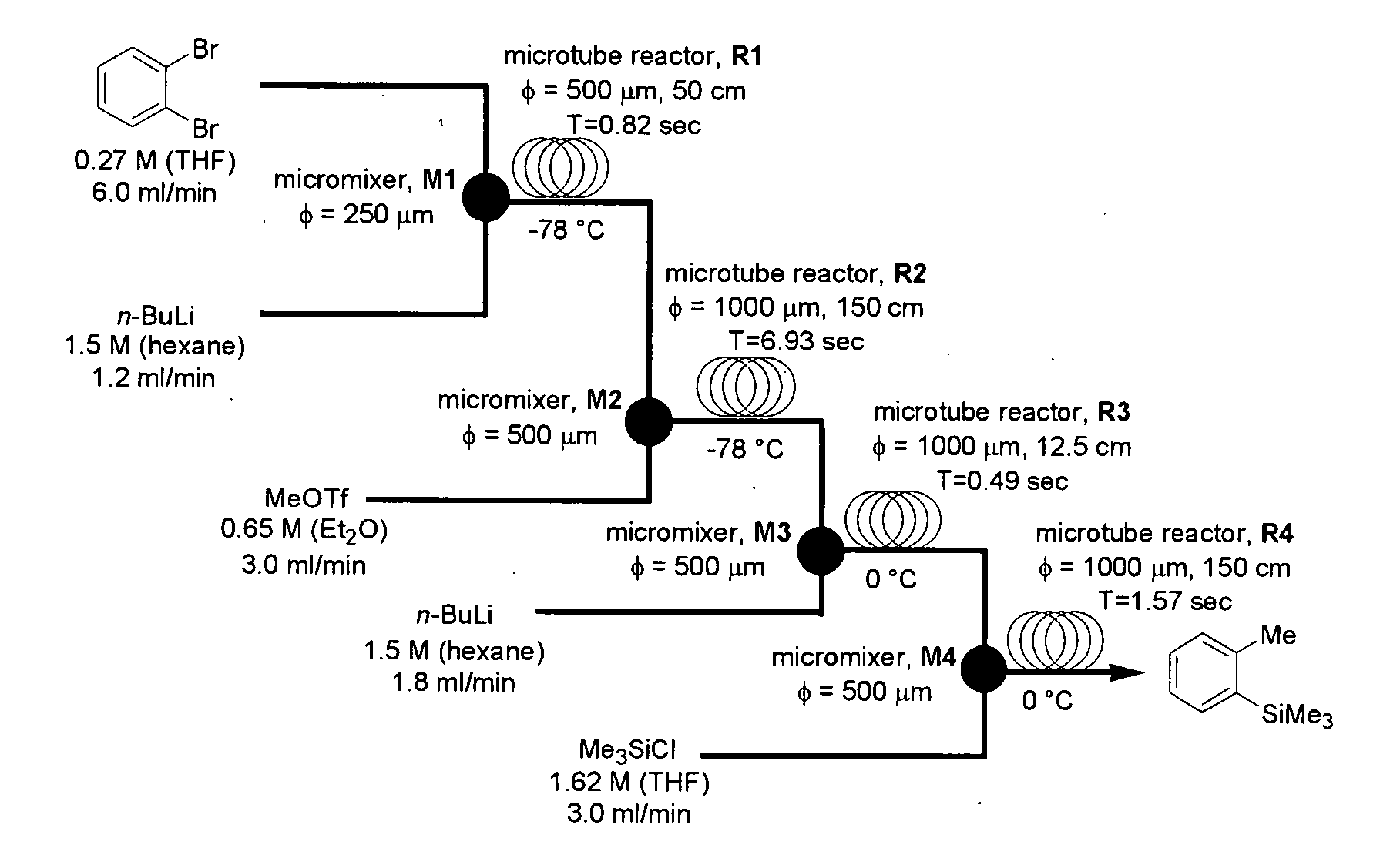
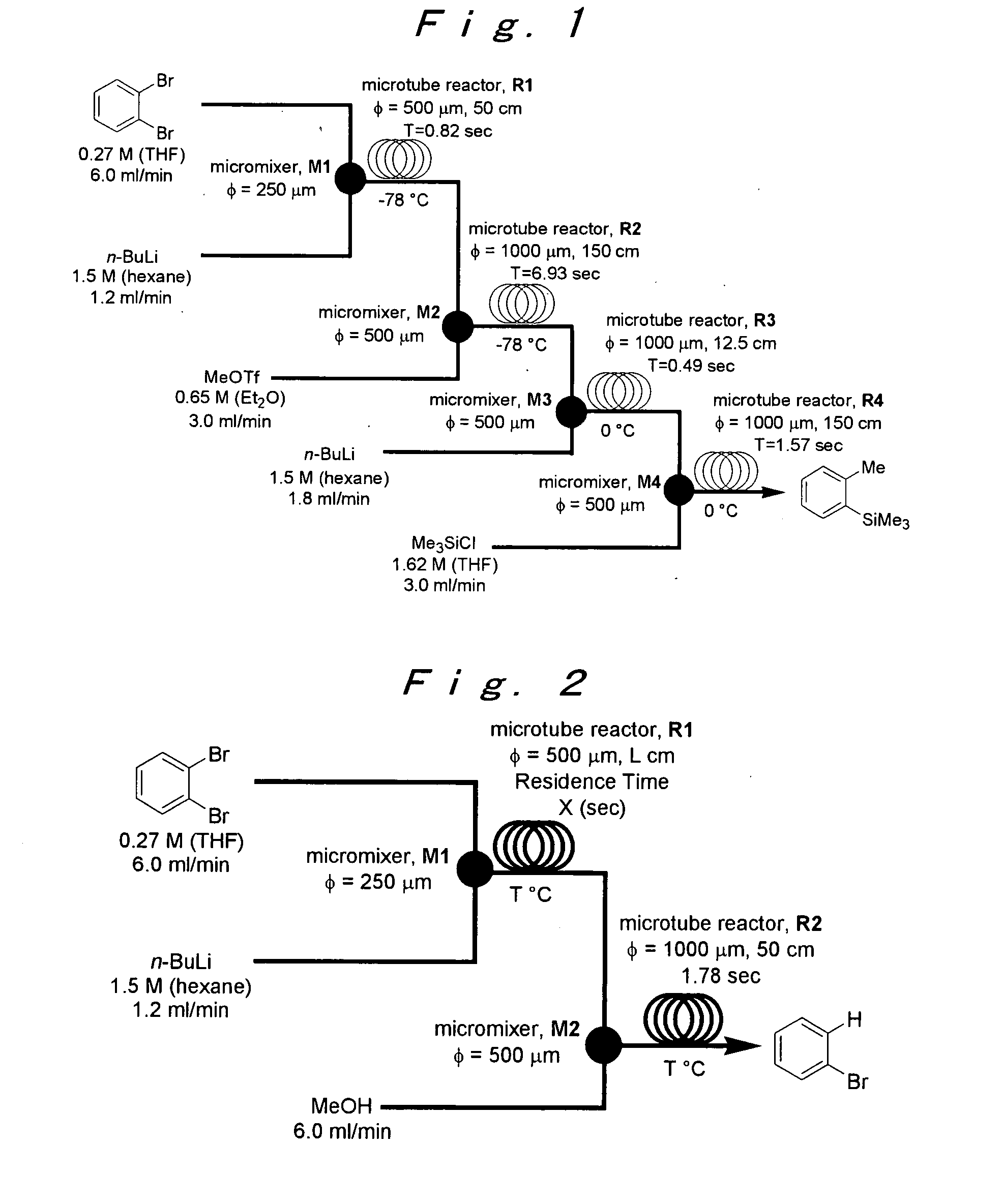
A method of producing an o-disubstituted aromatic compound, containing: continuously conducting at least the following steps (a) to (d):(a) a step of mono-lithiating one halogen atom of an o-dihaloaromatic compound, using a first microreactor;(b) a step of making the thus-obtained monolithiated product to react with an electrophilic compound, using a second microreactor, to obtain a monosubstituted-monohaloaromatic compound;(c) a step of lithiating the other halogen atom of the o-dihaloaromatic compound, using a third microreactor; and(d) a step of making the thus-obtained lithiated product successively to react with an electrophilic compound, using a forth microreactor.
Owner:FUJIFILM CORP
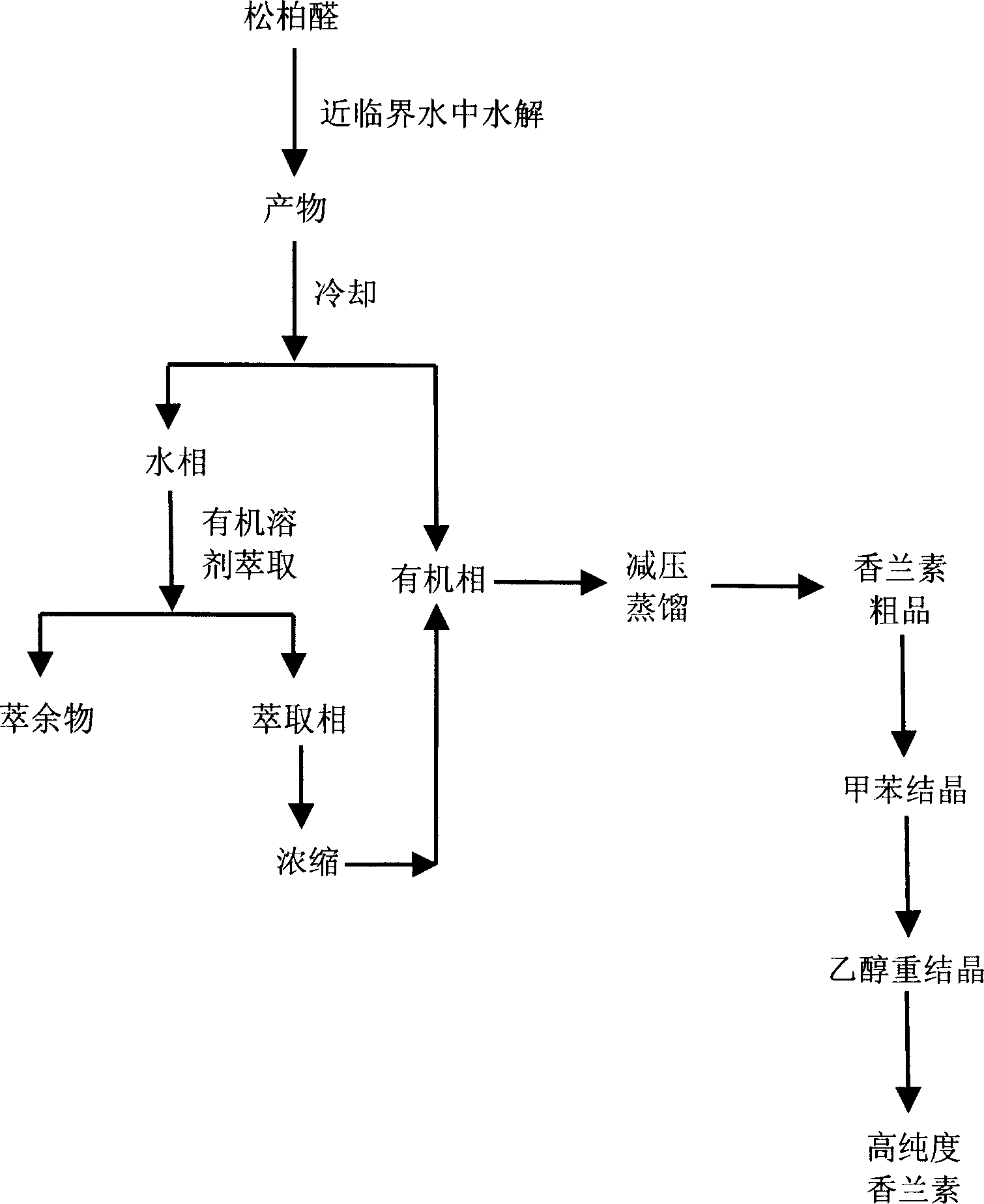
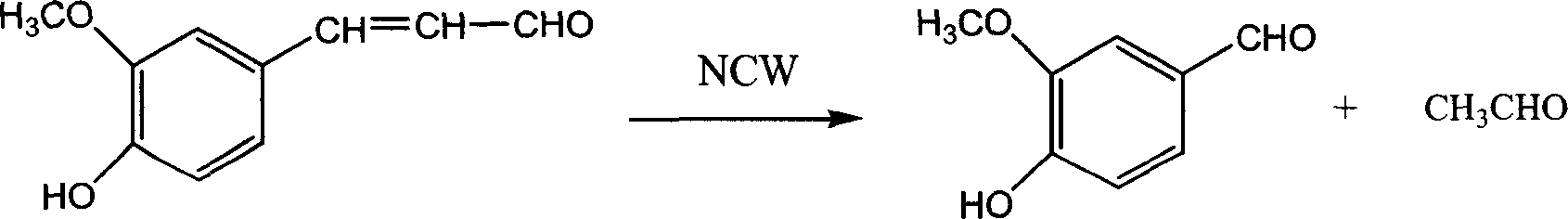
No-catalyst ferulaldhyde hydrodizing process to prepare vanillin in near critical water medium
The present invention discloses no-catalyst ferulaaldehyde hydrolyzing process to prepare vanillin in near critical water medium. The process includes the following steps: 1. adding deionized water and ferulaaldehyde in the mass ratio of 1-6 into a high pressure reactor, heating at normal pressure and while stirring to boil, and opening the exhaust valve for 2-5 min; 2 further heating to 200-320 deg.c for hydrolysis for 0.5-8 hr; 3 cooling the hydrolysate, separating into water phase and organic phase, extracting the water phase with organic solvent and distilling the extracted phase before merging with the organic phase; 4 decompression distilling the merged organic phase to obtain crude vanillin product, crystallizing in toluene and re-crystallizing in ethanol to obtain high purity vanillin product. The process is simple, high in yield and environment friendly, and the obtained vanillin product has high quality.
Owner:ZHEJIANG UNIV
Method for producing guerbet alcohols
Guerbet alcohols of the formula (I)CH3(CH2)nCHR1CH2OH (I)wherein R1 is a linear alkyl radical having n-1 carbon atoms and n is a number of from 5 to 11 are produced by a process which comprises contacting a fatty alcohol of the formula (II)R2OH (II)wherein R2 is a linear alkyl radical having from 6 to 12 carbon atoms with a carbonyl compound and an alkall metal hydroxide. The use of heavy metal catalysts usually associated with the production of Guerbet alcohols is avoided thereby reducing the potential for heavy metal pollution of the environment.
Owner:COGNIS DEUT GMBH & CO KG
Selective process for conversion of syngas to ethanol
ActiveUS8288594B2Organic compound preparationOxygen compounds preparation by reductionSyngasUnit operation
Owner:ALBEMARLE CORP
Method for producing alkyl-substituted butenols
Alkyl-substituted butenols having the formula (I):R1—CH2—CH═CR2—CH2OH (I)wherein R1 is a saturated or olefinically unsaturated alkyl or cycloalkyl group having from 4 to 16 carbon atoms and wherein R1 is optionally substituted by an alkyl, cycloalkyl, aryl or alkaryl having up to 12 carbon atoms; R2 is hydrogen or an alkyl group having from 1 to about 6 carbon atoms are produced by a process which comprises: (1) reacting an aldehyde of the formula (II):R1—CH2—CHO (II)wherein R1 has the same meaning as in formula (I), with the corresponding lower aldehyde to form an unsaturated aldehyde in an inert organic solvent; (2) continuously contacting an optionally calcined copper / zinc catalyst with the unsaturated aldehyde under isothermal conditions at temperatures of from about 45 to about 60° C. and under a hydrogen pressure of from 1 to about 300 bar.
Owner:KAO CORP
Process for producing optically active propargyl alcohols
Owner:SUMITOMO CHEM CO LTD
Application of chiral critical clusters to assymetric synthesis
InactiveUS6486355B1Amino preparation from aminesOrganic compound preparationSolvent moleculeHydrogen
Disclosed is a composition, a method of making and a method of using critical clusters for asymmetric synthesis using substantially optically-pure chiral solvent molecules in a supercritical fluid. The solvent molecules are capable of forming a multipoint hydrogen bonded solvate as they encage at least one solute molecule. The encaged solute molecule is capable of reacting to form an optically active chiral center. In another aspect, there is disclosed a method of directing the position of bonding between a solute molecule and a ligand involving encaging the solute molecule and the ligand with polar solvent molecules in a supercritical fluid under conditions of temperature and pressure sufficient to change electric charge distribution in the solute molecule. In yet another aspect, disclosed is a method of making pharmaceutical compounds involving encaging a solute molecule, which is capable of forming a chiral center, and a ligand with polar solvent molecules in a supercritical fluid under conditions of temperature and pressure sufficient to change electric charge distribution of the solute molecule. The solute molecule and ligand are then reacted whereby the ligand bonds to the solute molecule forming a chiral center. Also disclosed is a method for racemic resolution using critical clusters involving encaging racemic mixtures of solute molecules with substantially optically-pure chiral solvent molecules in a supercritical fluid under conditions of temperature and pressure sufficient to form critical clusters. The solvent molecules are capable of multipoint hydrogen bonding with the solute molecules. The encaged solute molecules are then nonenzymatically reacted to enhance the optical purity of the solute molecules.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
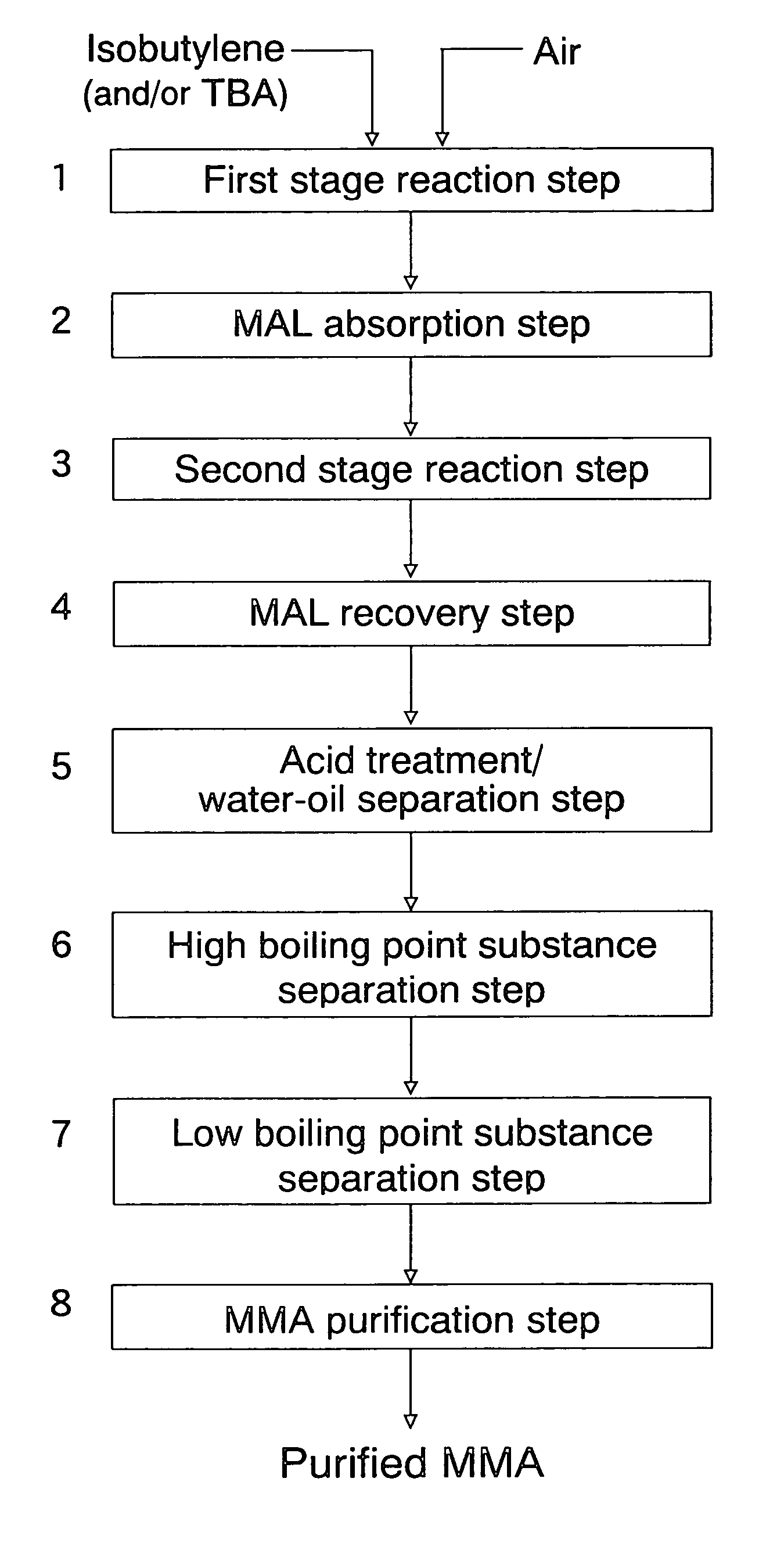
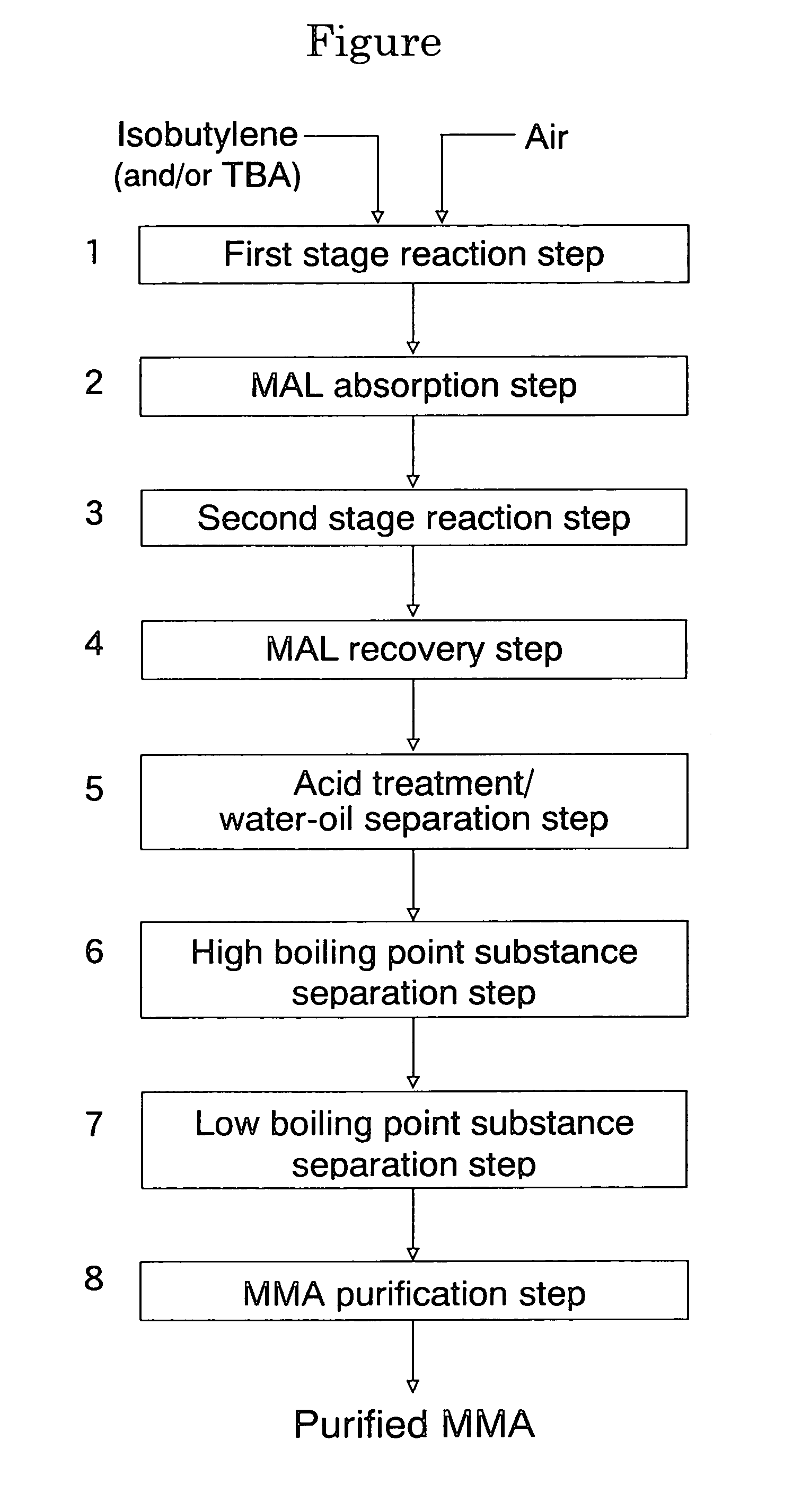
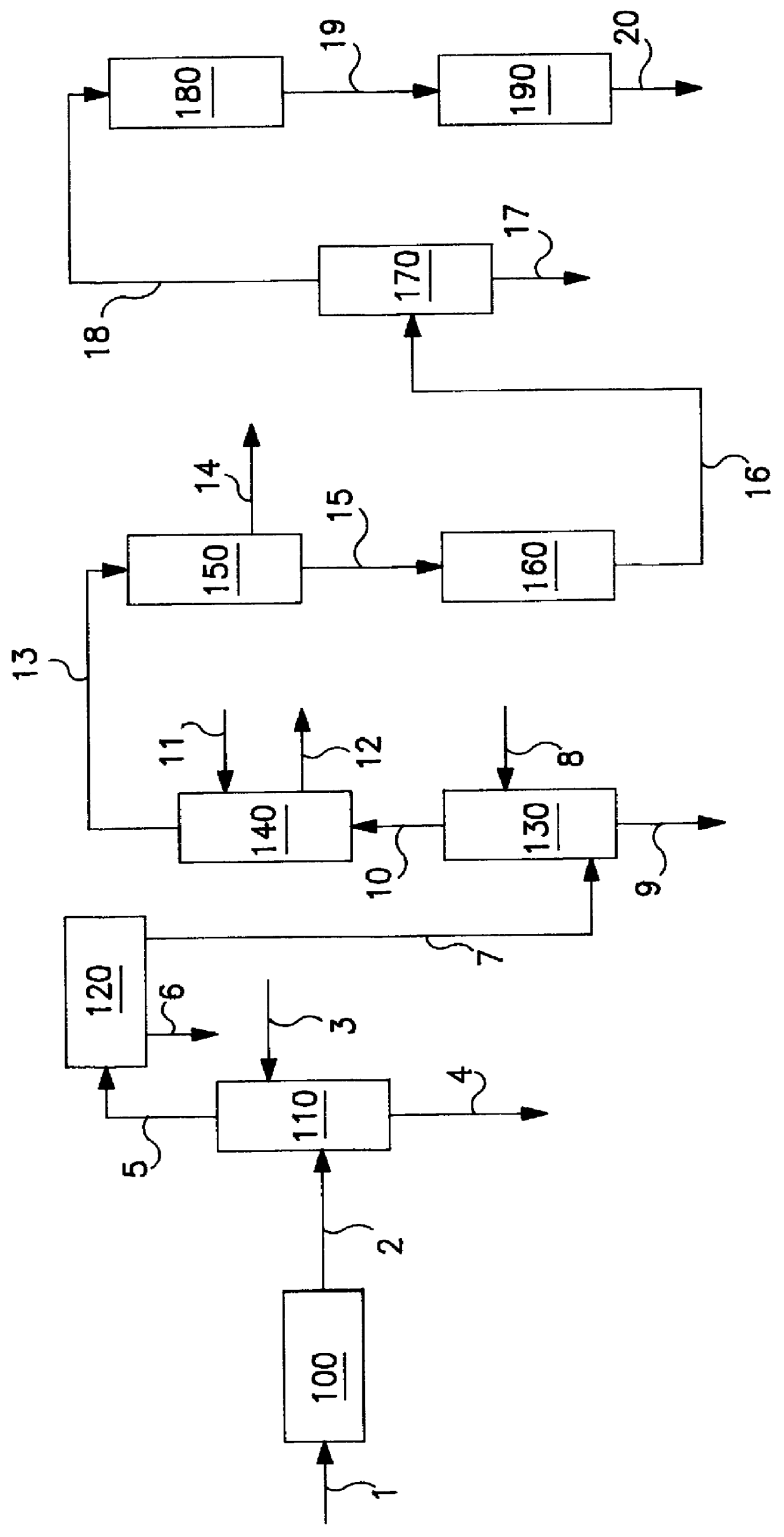
Process for producing isobutylene and methanol
InactiveUS20030009063A1Loss of methanol contained in the side cutIncrease volumeOrganic compound preparationOrganic chemistry methodsDecompositionDistillation
A process for producing isobutylene and methanol, comprising decomposing methyl-tert-butylether into isobutylene and methanol, and separating into isobutylene and methanol, thereby individually recovering isobutylene and methanol, the process comprising the following steps: a first step of subjecting methyl-tert-butylether to decomposition in the presence of a solid acid catalyst; a second step of washing the resultant with water to separate into an oil layer and a water layer; a third step of subjecting the oil layer to distillation to obtain a fraction from the top of the distillation column and a fraction from the bottom thereof; and a fourth step of subjecting the water layer to distillation to obtain a fraction containing methanol from the top of the distillation column, a fraction containing water from the bottom thereof and a fraction rich in tert-butanol from a side cut thereof, and then recycling a part or whole of the fraction from the side cut to the first step.
Owner:SUMITOMO CHEM CO LTD
Process for producing isobutylene and methanol
InactiveUS6696612B2Loss of methanol contained in the side cutIncrease volumeOrganic compound preparationOrganic chemistry methodsDecompositionDistillation
A process for producing isobutylene and methanol, comprising decomposing methyl-tert-butylether into isobutylene and methanol, and separating into isobutylene and methanol, thereby individually recovering isobutylene and methanol, the process comprising the following steps:a first step of subjecting methyl-tert-butylether to decomposition in the presence of a solid acid catalyst;a second step of washing the resultant with water to separate into an oil layer and a water layer;a third step of subjecting the oil layer to distillation to obtain a fraction from the top of the distillation column and a fraction from the bottom thereof; anda fourth step of subjecting the water layer to distillation to obtain a fraction containing methanol from the top of the distillation column, a fraction containing water from the bottom thereof and a fraction rich in tert-butanol from a side cut thereof, and then recycling a part or whole of the fraction from the side cut to the first step.
Owner:SUMITOMO CHEM CO LTD
Ethane, propane selective oxidation catalyst high in activity, and preparation method and applications thereof
InactiveCN108160068AImprove catalytic performanceGood dispersionOrganic compound preparationOxygen compounds preparation by hydrocarbon oxidationVanadium dopingSynthesis methods
The invention discloses a vanadium doped mesoporous nanometer SiO2 particle catalyst used for ethane and propane selective oxidation, and a preparation method thereof, and belongs to the technical field of heterogeneous catalysis. Mesoporous nanometer SiO2 particle is taken as a carrier of the catalyst, vanadium is taken as an active component, vanadium oxides are doped into the mesoporous nanometer SiO2 particle skeleton. The preparation method is simple; the specific surface area is large; the channel structure is abundant; in preparation, one-step synthesis method is adopted, and in-suit doping of the active component vanadium is carried out; the preparation method is simple; preparation cycle is short; the structure of the catalyst active component obtained via one-step synthesis method is more stable; the catalyst possesses excellent ethane, propane selective oxidation performance, is high in ethane propane conversion rate, and high in product selectivity.
Owner:SHENYANG NORMAL UNIV
Method of producing an o-disubstituted aromatic compound, and method of producing a monosubstituted-monohaloaromatic compound
A method of producing an o-disubstituted aromatic compound, containing: continuously conducting at least the following steps (a) to (d):(a) a step of mono-lithiating one halogen atom of an o-dihaloaromatic compound, using a first microreactor;(b) a step of making the thus-obtained monolithiated product to react with an electrophilic compound, using a second microreactor, to obtain a monosubstituted-monohaloaromatic compound;(c) a step of lithiating the other halogen atom of the o-dihaloaromatic compound, using a third microreactor; and(d) a step of making the thus-obtained lithiated product successively to react with an electrophilic compound, using a forth microreactor.
Owner:FUJIFILM CORP
Mixed metal oxide containing sulfur
InactiveUS20060269462A1Organic compound preparationHeterogenous catalyst chemical elementsSulfurDecomposition
Owner:EXXONMOBIL CHEM PAT INC
Mixed metal oxide containing sulfur
InactiveUS7173158B2Organic compound preparationHeterogenous catalyst chemical elementsDecompositionSulfur
Owner:EXXONMOBIL CHEM PAT INC
Fluidizable carbon catalysts
InactiveUS20070299277A1Good fluidization effectHigh rateOrganic compound preparationPreparation by carbon monoxide or formate reactionPolymer sciencePtru catalyst
Disclosed are fluidizable catalysts comprising carbonized, polysulfonated vinylaromatic polymer particles. These carbonized polymer particles can be active catalysts by themselves or can act as supports for active catalyst components. These novel catalysts show excellent fluidization behavior over a wide range of gas velocities. Also disclosed are processes for making fluidizable catalysts, for fluidizing these catalysts, and for the preparation of carbonylation products with these catalysts.
Owner:EASTMAN CHEM CO
Direct catalytic conversion of cellulose materials to ethanol
InactiveUS7816568B2Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCelluloseDistillation
Catalytic reactions conducted during acid digestion of cellulose materials, including paper, a wide range of grasses including prairie grass, switch grass, pine wood sawdust, bagasse dried after sugar cane processing, cotton, waste cellulose products and starch materials, are taught for direct conversion to ethanol. The cellulose material is thoroughly wet in concentrated sulfuric acid in the presence of transition metal complexes possessing a degree of symmetry. Ethanol formed during the reaction can be removed by distillation affording a continuous process.
Owner:CARTER TECH
Process for purifying ethanol
InactiveUS20120035400A1Organic compound preparationOxygen compounds preparation by reductionAcetic acidAcetaldehyde
In one embodiment, the invention is to a process for producing ethanol. The process comprises the step of hydrogenating an acetic acid feed stream in a first reactor to form a crude ethanol product. The crude ethanol product may comprise, among other components, ethanol, acetaldehyde, and residual acetic acid. The process further comprises the step of recovering acetaldehyde from the crude ethanol product. The process further comprises the steps of feeding a first portion of the recovered acetaldehyde to the first reactor and reacting the first portion of the recovered acetaldehyde in the first reactor to form additional ethanol. In addition, the inventive process may comprise the steps of feeding a second portion of the recovered acetaldehyde to a second reactor and reacting the second portion of the recovered acetaldehyde in the second reactor to faun an additional product.
Owner:CELANESE INT CORP
Process for the production of primary alcohols
InactiveUS7098369B2High selectivityPreparation by oxo-reaction and reductionOrganic compound preparationAlcoholHydrogen
A process for producing primary alcohols from secondary alcohols and / or tertiary alcohols and / or ketones, wherein the process comprises reacting a compound selected from a secondary alcohol, a tertiary alcohol, a ketone, or mixtures thereof, with carbon monoxide and hydrogen in the presence of a catalyst based on:(i) a source of Group VIII metal,(ii) a bidentate ligand having the general formula (I):R1R2M1-R-M2R3R4 (I)wherein M1 and M2 are independently P, As or Sb;R1 and R2 together represent a bivalent substituted or unsubstituted cyclic aliphatic group whereby the two free valencies are linked to M1; R3 and R4 independently represent a substituted or unsubstituted hydrocarbyl group, or together represent a bivalent or non-substituted cyclic group whereby the two free valencies are linked to M2; andR represents a bivalent aliphatic bridging group; and(iii) an acid having a pKa of 3 or less which is in excess over the Group VIII metal.
Owner:SHELL OIL CO
Popular searches
Carboxylic preparation by oxidation Carbonyl compound separation/purification Metal/metal-oxides/metal-hydroxide catalysts Preparation by hydrolysis Essential-oils/perfumes Preparation by carbon monoxide reaction Base-materials Preparation by oxygen reduction Carboxylic compound preparation Halogenated hydrocarbon preparation
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com