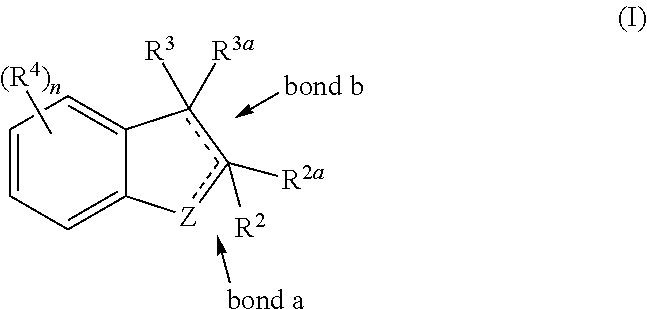
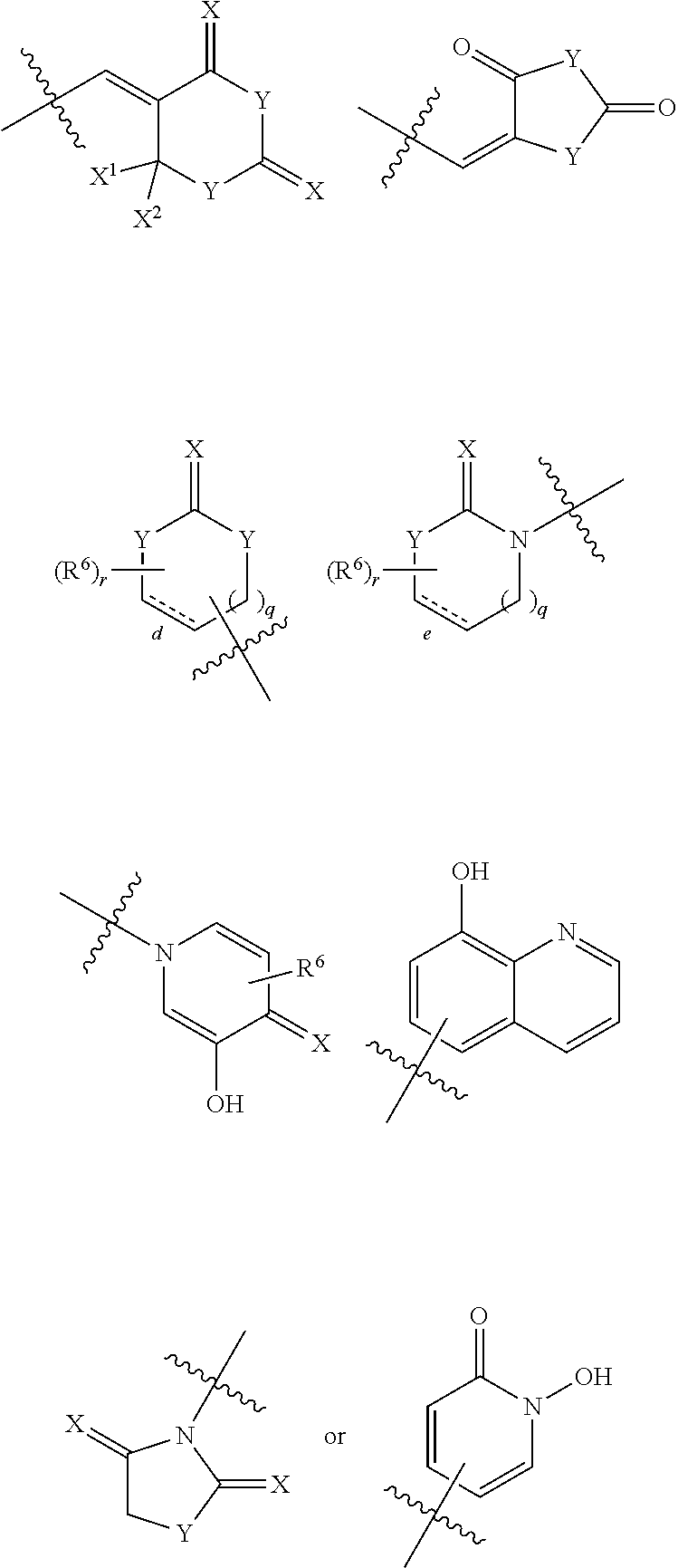
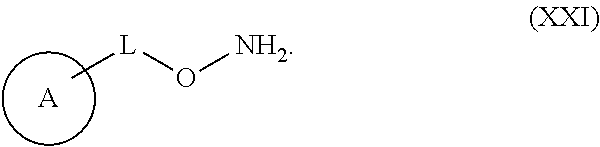IDO Inhibitors
a technology of indoleamine and inhibitors, which is applied in the direction of antiinfectives, drug compositions, immunological disorders, etc., can solve the problem that the fetus cannot fully explain the survival of the fetal allogra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Method A
Syntheses of Dithiocarbamates with Variations in S-Alkyl Groups (Scheme 1)
[0702]
[0703]A solution of tryptamine 1 (1.0 equiv) in anhydrous CH2Cl2 (10 mL) at 0° C. was treated sequentially with triethylamine (1.1 equiv) then carbon disulfide (1.1 equiv) and stirred for 30 min. After this time, alkyl bromide 2 (1.2 equiv unless indicated otherwise) was added and the reaction was allowed to warm to room temperature and stirred overnight. The reaction mixture was then poured into 1 M H2SO4 and extracted with EtOAc (3×10 mL). The combined organic layers were washed with brine, dried over anhydrous Na2SO4, filtered and concentrated. Purification by flash chromatography (silica, EtOAc / hexanes) afforded the desired product. The dithiocarbamate product typically exists as a z 7:3 mixture of tautomers observed by 1H NMR, and are listed with spectral data.
example 2
Phenethyl 2-(1H-indol-3-yl)ethylcarbamodithioate [Compound 00001]
[0704]
[0705]The reaction of 1 with (2-bromoethyl)benzene (1.2 equiv) was performed as described in Method A. Purification by flash chromatography (silica, gradient of 12% EtOAc / Hexanes to 100% EtOAc) afforded product 00001 (0.148 g, 35%) as a yellow oil: 1H NMR (500 MHz, CDCl3) δ 8.04 (br s, 1H), 7.62 (d, J=7.5 Hz, 1H), 7.38 (d, J=8.0 Hz, 1H), 7.32-7.25 (m, 2H), 7.25-7.20 (m, 4H), 7.16-7.13 (m, 1H), 7.07-7.05 (m, 1H), 6.92 (br s, 1H), 4.11-4.06 (m, 2H), 3.48-3.45 (m, 2H), 3.13 (t, J=6.5 Hz, 2H), 2.97-2.94 (m, 2H) and signals due to a minor tautomer (ca. 31%): 3.78-3.74 (m), 3.59-3.55 (m), 3.10-3.08 (m), 3.05-3.01 (m); ESI MS m / z 341 [M+H]+, HPLC (Method 1) >99% (AUC), tR=13.9 min.
example 3
4-Fluorophenethyl 2-(1H-indol-3-yl)ethylcarbamodithioate [Compound 00003]
[0706]
[0707]The reaction of 1 with 4-fluorophenethyl bromide (0.6 equiv) was performed as described in Method A. Purification by flash chromatography (silica, gradient of 12% EtOAc / Hexanes to 100% EtOAc) afforded product 00003 (0.084 g, 31%) as a yellow oil: 1H NMR (500 MHz, CDCl3) δ 8.04 (br s, 1H), 7.63 (d, J=8.0 Hz, 1H), 7.39 (d, J=8.0 Hz, 1H), 7.24-7.13 (m, 4H), 7.08-7.04 (m, 1H), 7.00-6.94 (m, 2H), 6.92 (br s, 1H), 4.11-4.07 (m, 2H), 3.46-3.43 (m, 2H), 3.14 (t, J=7.0 Hz, 2H), 2.94-2.91 (m, 2H) and signals due to a minor tautomer (ca. 31%): 3.79-3.75 (m), 3.55-3.52 (m), 3.11-3.09 (m), 3.01-2.98 (m); ESI MS m / z 359 [M+H]+, HPLC (Method 1) 95.3% (AUC), tR=13.7 min.
PUM
| Property | Measurement | Unit |
|---|---|---|
| octanol-water partition coefficient | aaaaa | aaaaa |
| particle sizes | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com