Human Anti-tau antibodies
a technology of human anti-tau and binding molecules, which is applied in the direction of antibodies, depsipeptides, peptide/protein ingredients, etc., can solve the problems of the risk of tau-targeting active immunotherapy approaches
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Validation of Target and Binding Specificity of Human Tau-Antibodies
[0279]To validate tau as a recognized target of isolated antibodies direct ELISA assays were performed as described above. For the exemplary recombinant human NI-105.4A3 antibody 96 well microplates (Costar, Corning, USA) were coated with recombinant human tau (hTau40, rPeptide, Bogart, USA) diluted to a concentration of 3 μg / ml or with BSA in carbonate ELISA coating buffer (pH 9.6) and binding efficiency of the antibody was tested. The exemplary NI-105.4A3 antibody specifically binds to human tau by ELISA. No binding is observed to BSA (FIG. 10).
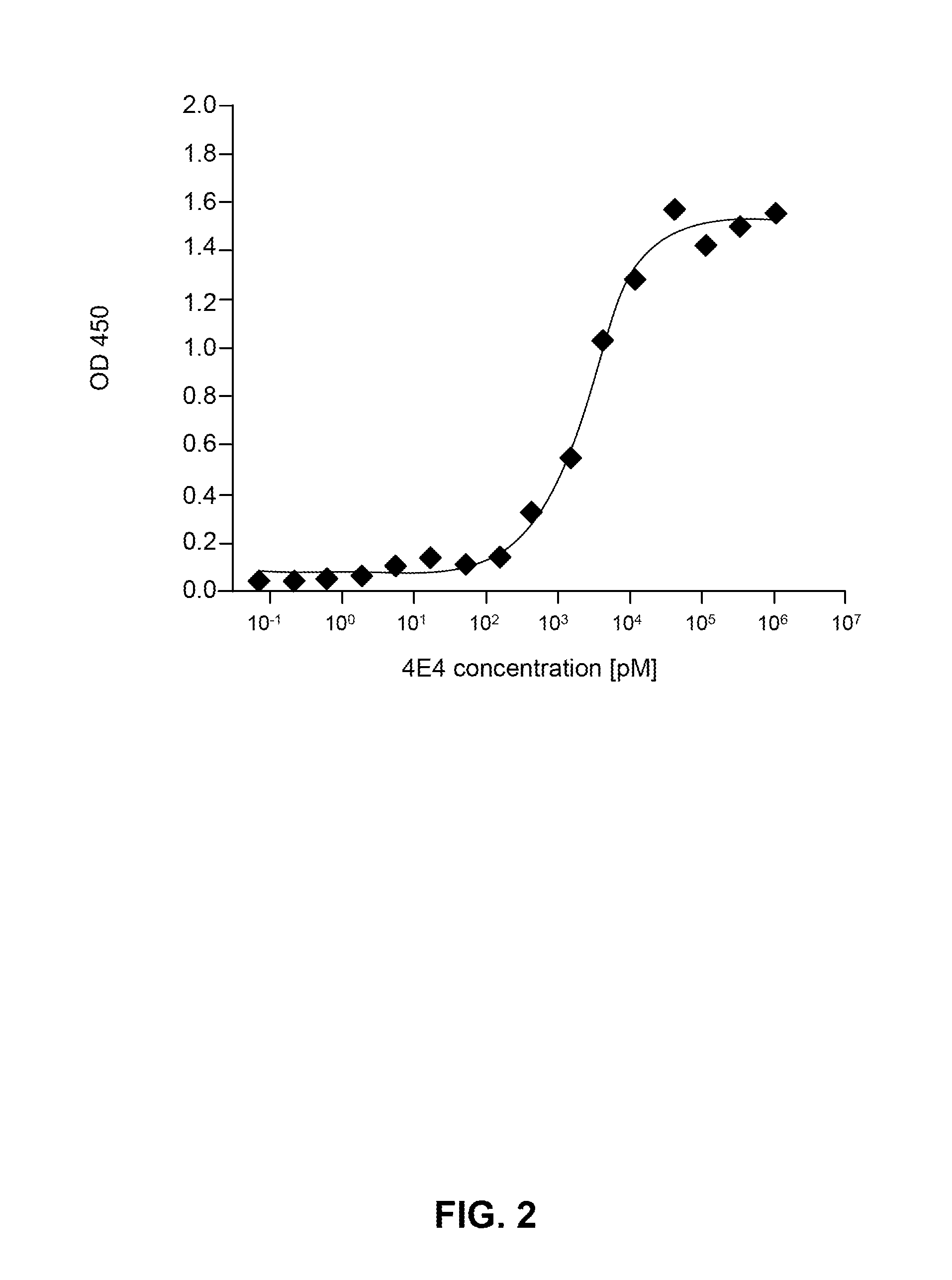
[0280]For a determination of the half maximal effective concentration (EC50) of the exemplary antibodies NI-105.4E4 and NI-105.24B2 additional direct ELISA experiments with varying antibody concentrations were performed. 96 well microplates (Costar, Corning, USA) were coated with recombinant human tau (hTau40, rPeptide, Bogart, USA) diluted to a concentration of 1 μg / ml (fo...
example 2
Recombinant Human Antibodies Binding Analysis to Recombinant Tau and Pathological Tau Extracted from AD Brain
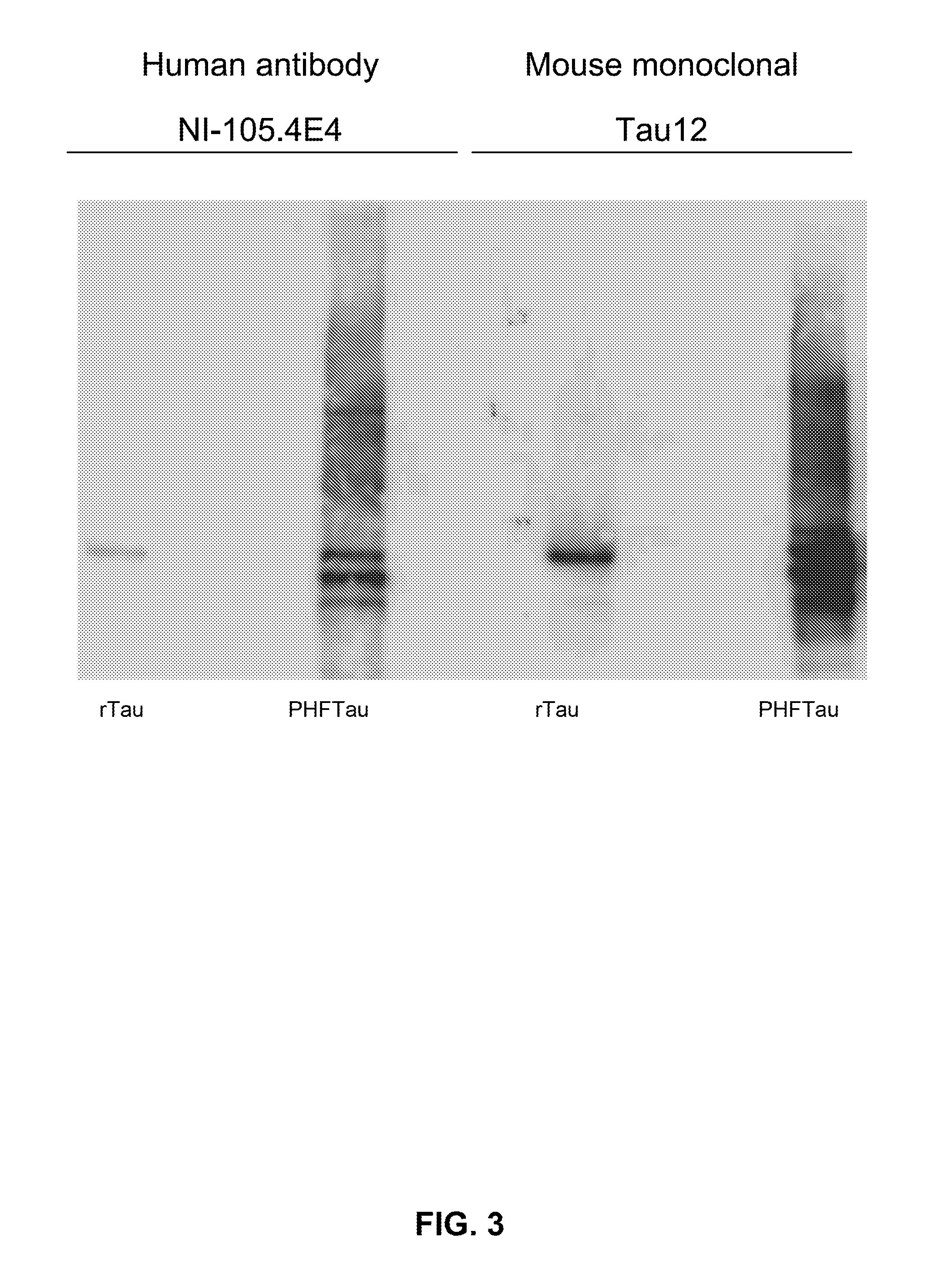
[0282]To determine the binding capacity of NI-105.4E4 and NI-105.24B2 to pathological tau species extracted from AD brain. SDS-PAGE and Western Blot analysis was performed as described in detail above. Blots were incubated overnight with primary antibodies NI-105.4E4 (human), NI-105.24B2 (human) or Tau12 (mouse monoclonal antibody, Covance, Calif., U.S.A.), followed by a HRP-conjugated goat anti-human IgGFcγ (for human antibodies) or a HRP-conjugated goat anti-mouse IgG secondary antibody.
[0283]Recombinant antibodies NI-105.4E4 (FIG. 3) and NI-105.24B2 (FIG. 8) recognize recombinant hTau40 as well as pathologically modified PHFTau extracted from AD brain on Western blot. As expected, control antibody Tau12 recognizes both tau species as well (FIG. 3).
[0284]Additionally, as discussed in Example 1 above, the half maximal effective concentration (EC50) of the exemplary antibody ...
example 3
Mapping of the NI-105.4E4 and NI-105.4A3 Binding Epitope on hTau40
[0285]A peptide array of 118 peptide sequences covering the full-length hTau40 (amino acids 1-441) with an overlap of 11 amino acids between two adjacent peptides was spotted on a nitrocellulose membrane (JPT Peptide Technologies GmbH, Berlin, Germany). Immunolabeling of antibodies as well as membrane regeneration were carried out according to manufacturer's instructions. To rule out non-specific binding of the detection antibody, the membrane was first probed by HRP-conjugated goat anti-human IgG omitting the primary antibody (FIG. 4B). After regeneration the membrane was probed with 100 nM recombinant NI-105.4E4 antibody. Bound antibody was detected using ECL and ImageQuant 350 detection (GE Healthcare, Otelfingen, Switzerland).
[0286]Two groups of adjacent peptide spots (peptide 83, 84 and 85; peptide 96 and 97) were specifically identified by NI105.4E4 (FIGS. 4A and A′), when compared to the detection antibody only...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com