Human antibodies against hepatitis c virus (HCV) and uses thereof
a technology of human antibodies and hepatitis c virus, which is applied in the field of human antibodies against hepatitis c virus (hcv) and its use, can solve the problems of slow development of hcv vaccines, ineffective vaccines against emerging strains, and inability to alleviate the problems faced by millions of chronic hcv carriers worldwide, and achieves low immunogenicity, localization to appropriate sites, and high purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Generation of Anti-HCV Monoclonal Antibodies
[0274]Mouse Immunizations, Hybridoma Production and Selection
[0275]Transgenic mice comprising human immunoglobulin genes generated as described above in the section entitled “Generation of Human Monoclonal Antibodies in HuMab Mice” and supplied by Medarex, Milpitas, Calif., were immunized with a soluble version of the HCV genotype 1a E2 envelope glycoprotein. The antigen was administered in combination with Freund's complete or RMI adjuvant. Mouse sera responses were monitored by enzyme linked immunosorbent assay (ELISA) to soluble E2 protein (1a E2-661). Splenic B cells were isolated from the immunized animal and fused to mouse myeloma (P3X-AG8.653) cells using standard spleen cells fusion methods. Clonal hybridomas were generated and screened using ELISA for production of antibody reactive to E2-661. Positive hybridomas were tested for equivalent reactivity to HCV genotype 1a and genotype 1b E2-661 by ELISA with decreasing amounts of hyb...
example 2
Antibody Characterization
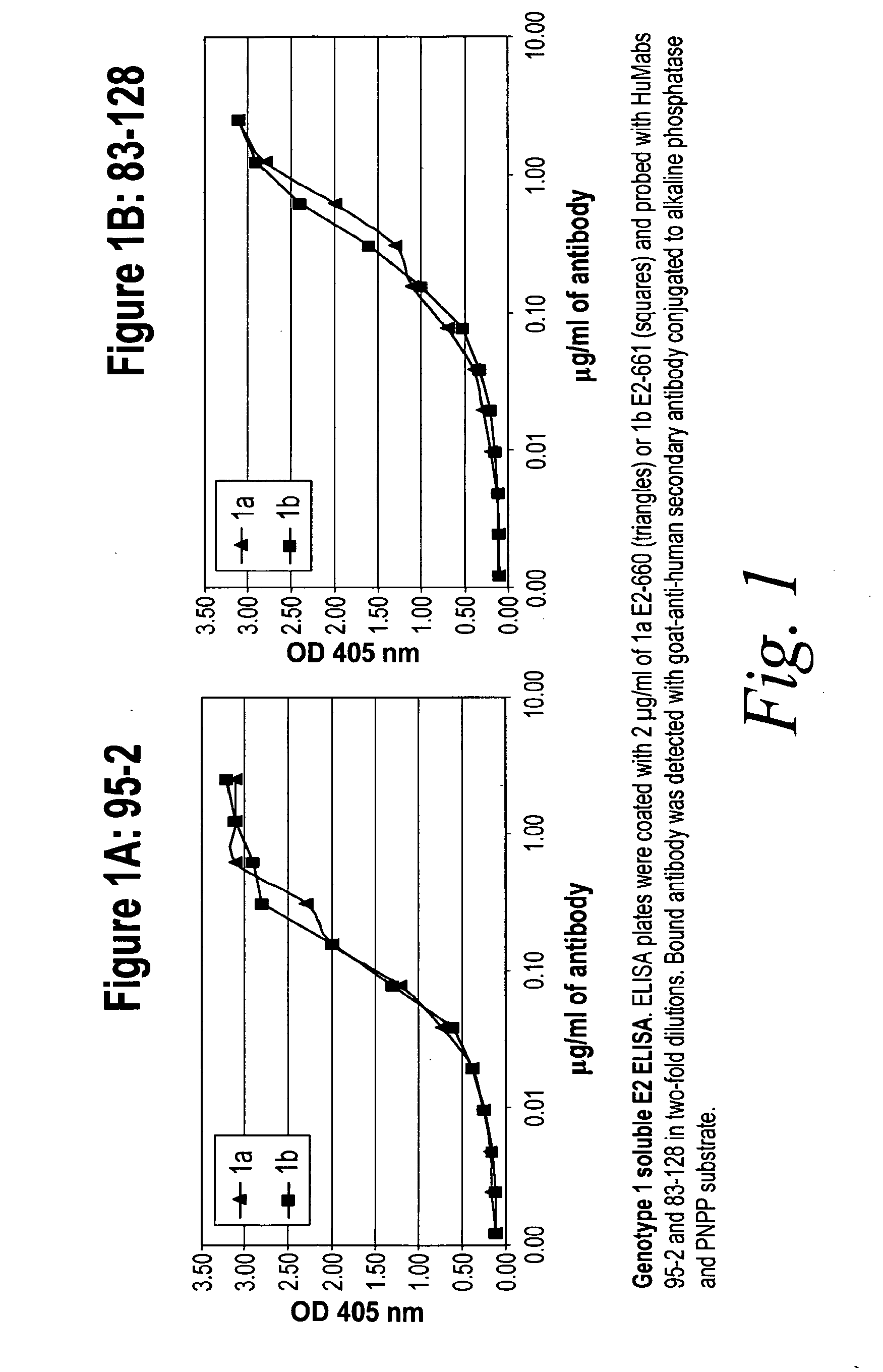
[0281]HCV Genotype 1a and 1b—Soluble E2 ELISA
[0282]The reactivity of human antibody 95-2 (FIG. 1A) and human antibody 83-128 (FIG. 1B) to soluble HCV genotype 1a E2-660 (triangles) and soluble HCV genotype 1b E2-661 (squares) was compared. ELISA plates were coated with 2 μg / ml of antigen and probed with antibody in two-fold dilutions. Bound antibody was detected with goat-anti-human secondary antibody conjugated to alkaline phosphatase and PNPP substrate. Both human antibodies, 95-2 and 83-128 were found to react equivalently to both genotypes (see FIG. 1). These experiments were also performed with the 95-14, 95-38 and 073-1 antibodies and all three recognized genotype 1a and 1b equivalently (data not shown).
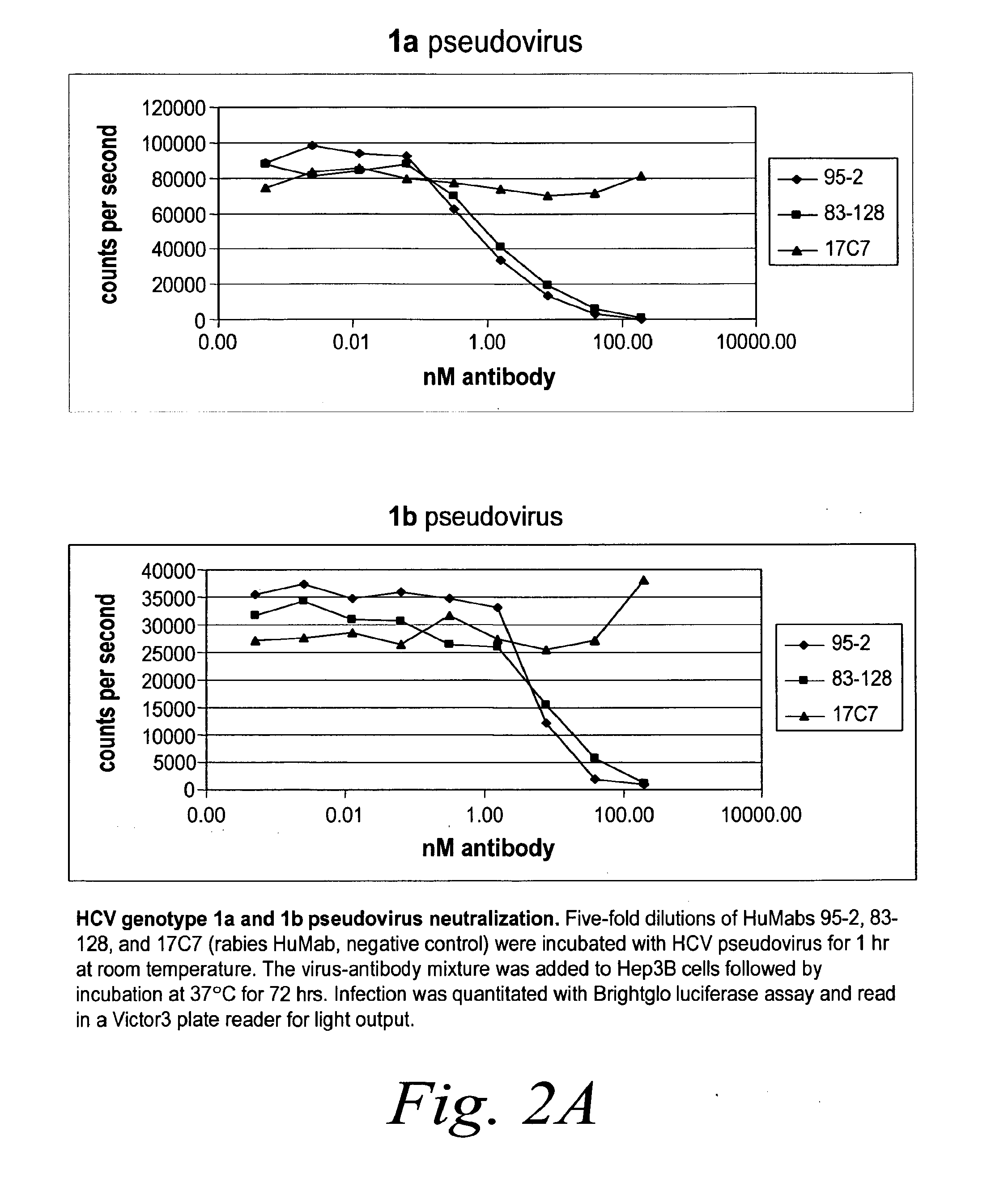
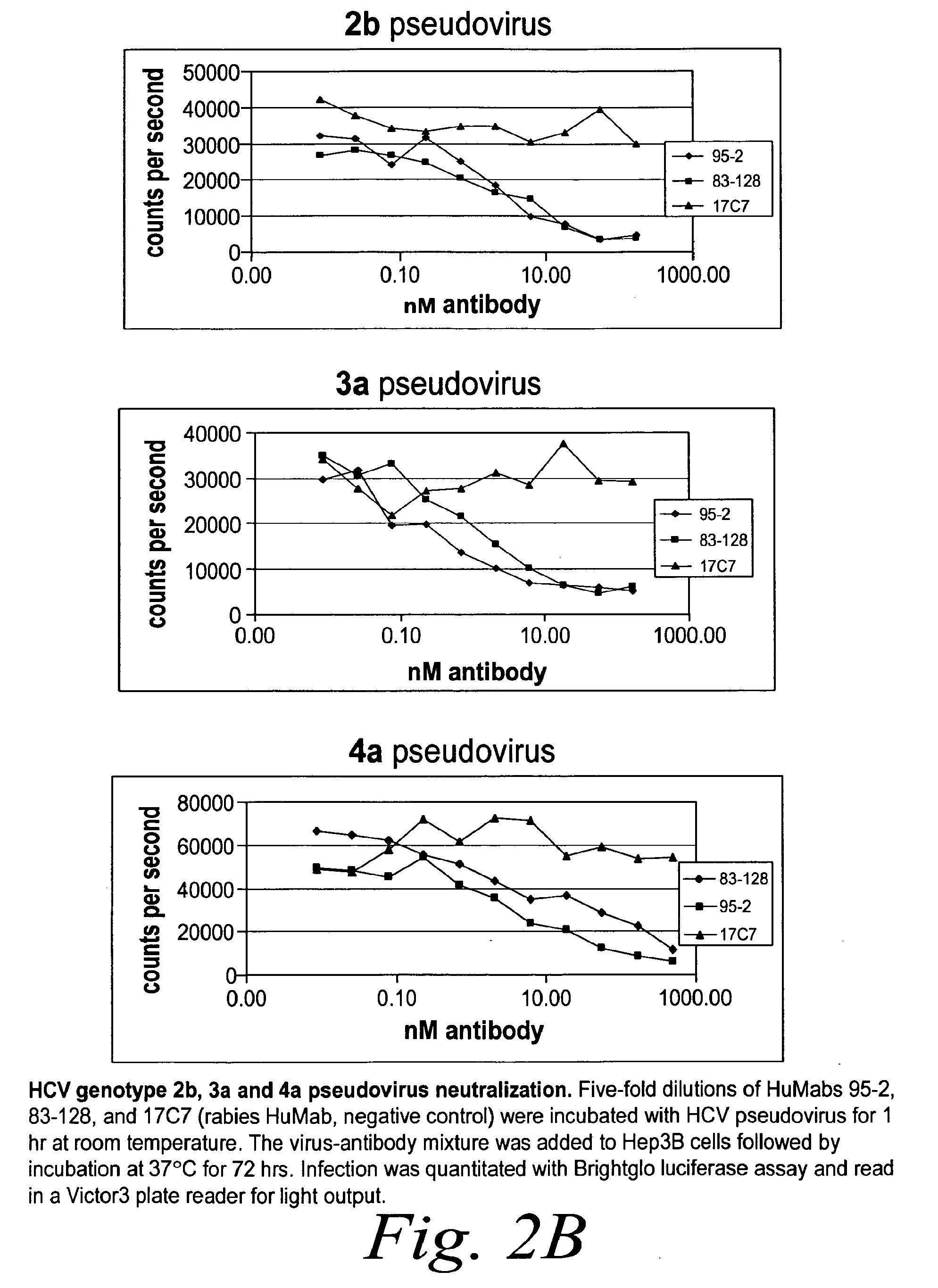
[0283]Neutralization of HCV Pseudovirus from Multiple Genotypes
[0284]The capacity of human antibodies 95-2 and 83-128 to neutralize both multiple genotypes of HCV pseudovirus was determined using Hep3B cells. Five-fold dilutions of antibody were incubat...
example 3
Epitope Determination
[0289]Determination of which Region of the E2 Protein Human Antibodies 83-128 and 95-2 Recognize
[0290]To determine which region of the E2 protein human antibodies 83-128 and 95-2 recognize, carboxy-terminal truncations of mammalian expressed E2 protein were captured by lectin ELISA and probed with 83-128 and 95-2 (see FIG. 5 for map of constructs and FIG. 6 for ELISA data). Based on the data, the epitope for these antibodies is within amino acids 412-464 of E2. As expected, all antibodies (95-14, 95-49, 95-62, 95-42, 95-58, 95-25, 95-43, 95-54, 95-2, 95-38, and 073-1) also mapped to this region of the E2 protein.
[0291]To further define the epitope, bacterially expressed fusion protein was used because the smaller pieces of E2 did not express well in the mammalian system. The purified proteins were coated on an ELISA plate and probed with human antibodies 83-128 and 95-2 (see FIG. 7 for map of constructs and FIG. 8 for ELISA data). Both human antibodies 83-128 an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com