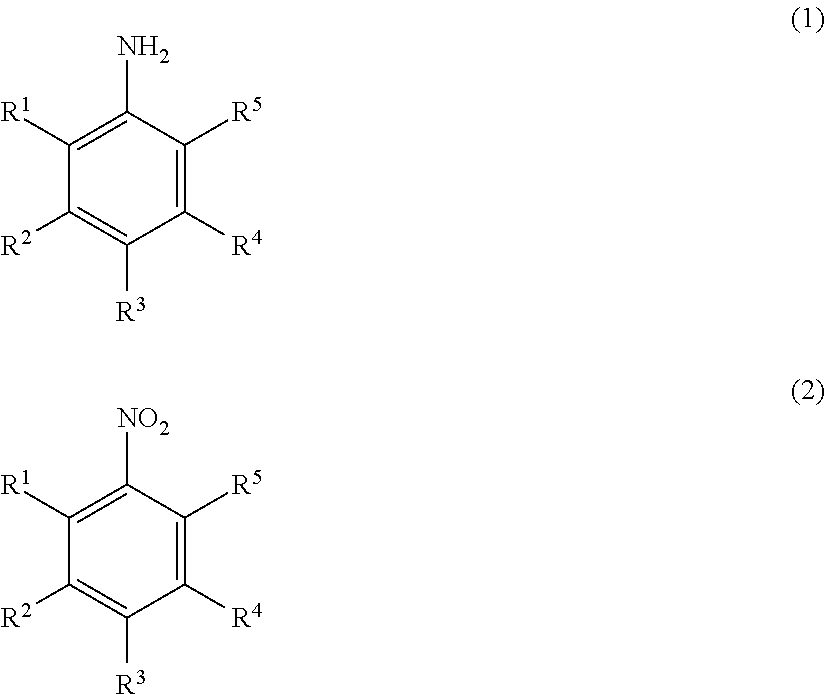
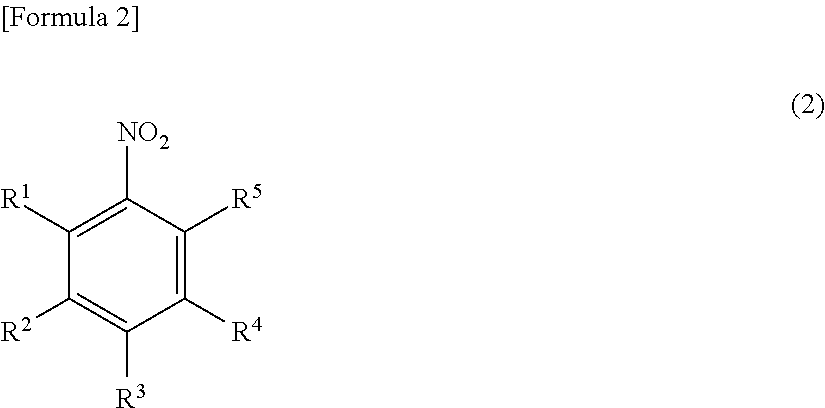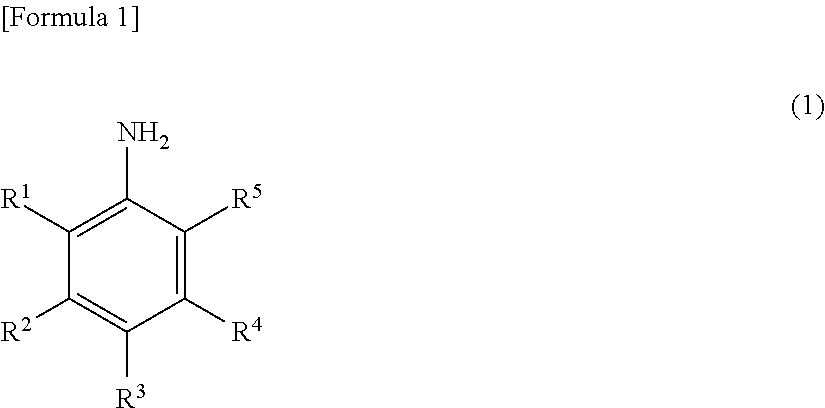Method for producing nitrobenzene compound
a nitrobenzene compound and nitrobenzene technology, applied in the field of nitrobenzene compound production, can solve the problems of no synthesis method for producing a 2,6-disubstituted nitrobenzene compound, and the target 2,6-disubstituted nitrobenzene compound cannot be obtained with good yield, etc., to achieve clean and excellent oxidant, easy to obtain, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of 2,6-Dichloronitrobenzene
[0161]1.32 g (4.0 mmol) of sodium tungstate dihydrate and 4.0 g (40 mmol) of concentrated sulfuric acid were added to a solution of 16.2 g (100 mmol) of 2,6-dichloroaniline in 120 ml of methanol, and the mixture was heated to 40° C. 30 ml (291 mmol) of a 30% hydrogen peroxide solution was added dropwise over 10 hours. The pH value at this time was 0.5. After the completion of the dropwise addition, the mixture was stirred at 40° C. for 9 hours. After the disappearance of the 2,6-dichloroaniline was confirmed by gas chromatography (area percentage method), a solution of 9.8 g (150 mmol) of 86% potassium hydroxide in 24.3 ml of methanol was added dropwise while the temperature of the reaction liquid was adjusted to 40° C. or less. After the completion of the dropwise addition, the mixture was stirred at room temperature for 2 hours, and the reaction was completed. After the completion of the reaction, 95 ml of toluene and 32 ml of water were added...
example 2
Production of Methyl 3-Chloro-2-nitrobenzoate
[0163]A solution of 5.3 g (16.2 mmol) of sodium tungstate dihydrate and 3.2 g (32.3 mmol) of concentrated sulfuric acid in 53 ml of methanol was heated to 40° C., and a solution of 15 g (80.8 mmol) of methyl 2-amino-3-chlorobenzoate in 13 ml of methanol, and 33.0 ml (323 mmol) of a 30% hydrogen peroxide solution were simultaneously added dropwise over 10 hours. The pH value at this time was 0.5. After the completion of the dropwise addition, the mixture was stirred at 40° C. for 2 hours. After the disappearance of the methyl 2-amino-3-chlorobenzoate was confirmed by HPLC analysis (area percentage method), 30 ml of toluene and 8.3 ml (80.8 mmol) of a 30% hydrogen peroxide solution were added to the reaction liquid, and 19.9 g (88.9 mmol) of a 25% aqueous potassium hydroxide solution was further added dropwise so that the temperature was 30° C. or less. The mixture was stirred at room temperature for 12 hours, and the reaction was completed...
example 3
Production of 2,6-Dichloronitrobenzene
[0165]0.66 g (2.64 mmol) of tungstic acid and 0.40 g (1.23 mmol) of tetrabutylammonium bromide were added to a suspension of 2.0 g (12.3 mmol) of 2,6-dichloroaniline in 6 ml of water, and the mixture was heated to 40° C. 4.9 g (43.1 mmol) of a 30% hydrogen peroxide solution was added. The pH value at this time was 1.5. Then, the mixture was stirred at 40° C. for 16 hours. After the disappearance of the 2,6-dichloroaniline was confirmed by gas chromatography (area percentage method), 3 ml of methanol and 3.49 g (30.8 mmol) of a 30% hydrogen peroxide solution were added, and 2.8 g (12.3 mmol) of a 25% aqueous potassium hydroxide solution was added dropwise while the temperature of the reaction liquid was adjusted to 40° C. or less. After the completion of the dropwise addition, the mixture was stirred at room temperature overnight, and the reaction was completed. After the completion of the reaction, 30 ml of toluene and 20 ml of water were added,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com