Method of Treating Cancer and Bone Cancer Pain
a bone cancer and cancer technology, applied in the field of cancer treatment, can solve the problems of abnormal deposition of unstructured bone, morbidity and mortality in crpc, and modest improvement in survival, so as to prolong the overall survival of patients and prevent bone metastases.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiments
[0048]In one embodiment the compound of Formula I is the compound of Formula Ia:
or a pharmaceutically acceptable salt thereof, wherein:
[0049]R1 is halo;
[0050]R2 is halo; and
[0051]Q is CH or N.
[0052]In another embodiment, the compound of Formula I is Compound 1:
or a pharmaceutically acceptable salt thereof. As indicated previously, compound I is referred to herein as N-(4-{[6,7-bis(methyloxy)quinolin-4-yl]oxy}phenyl)-N′-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide. WO 2005 / 030140 discloses Compound 1 and describes how it is made (Example 12, 37, 38, and 48) and also discloses the therapeutic activity of this compound to inhibit, regulate and / or modulate the signal transduction of kinases, (Assays, Table 4, entry 289). Example 48 is on paragraph [0353] in WO 2005 / 030140.
[0053]In other embodiments, the compound of Formula I, Ia, or Compound 1, or a pharmaceutically acceptable salt thereof, is administered as a pharmaceutical composition, wherein the pharmaceutical composition additio...
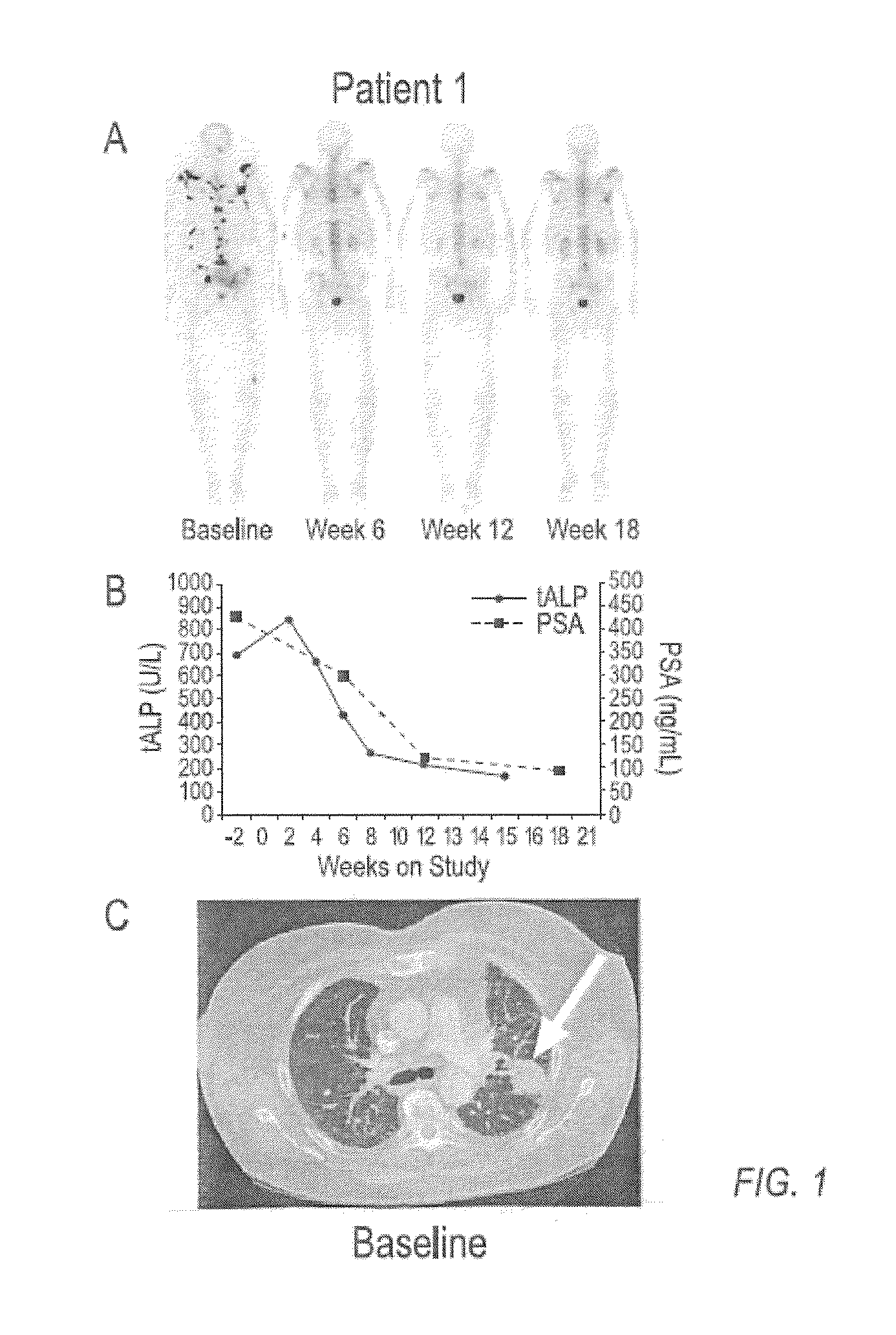
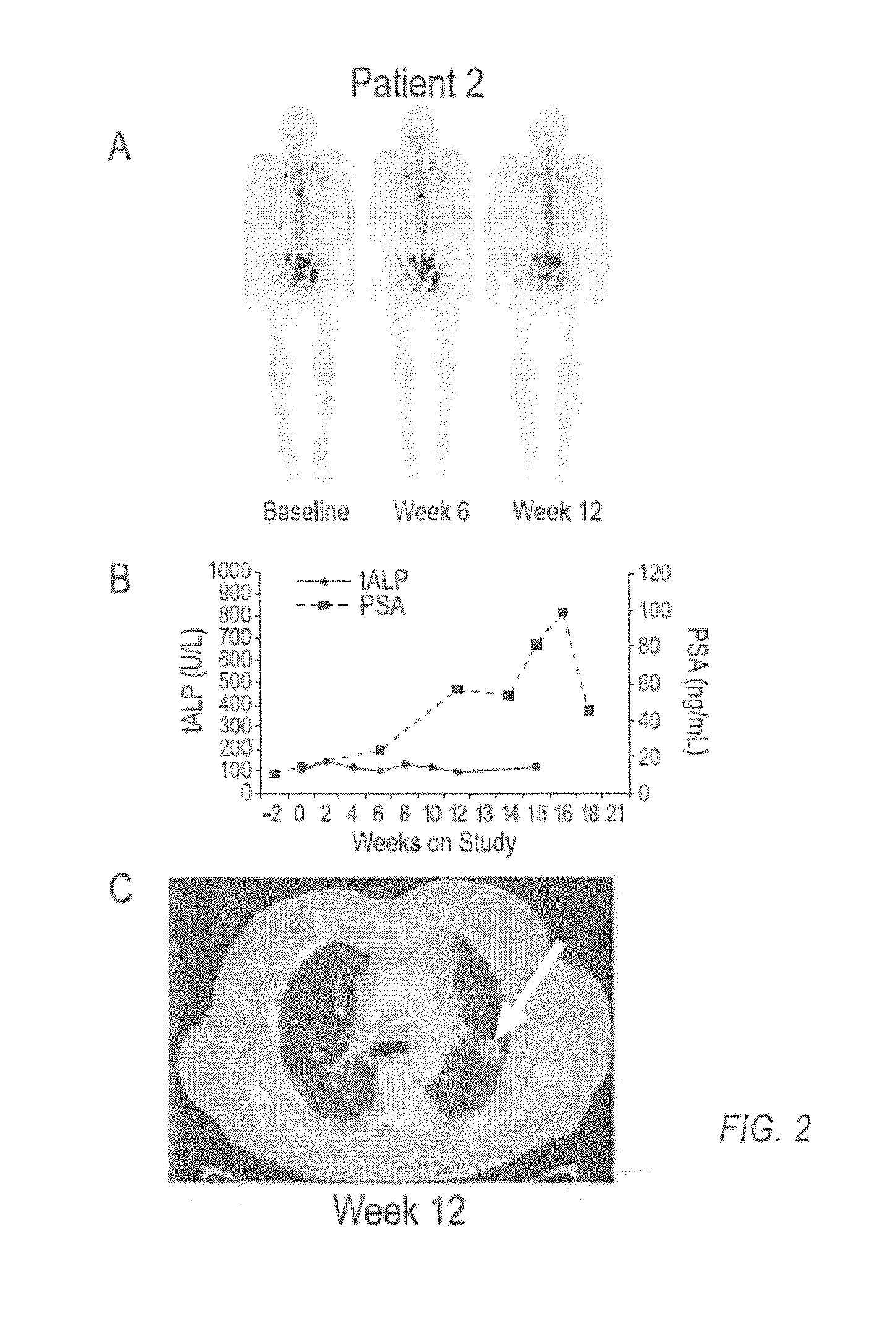
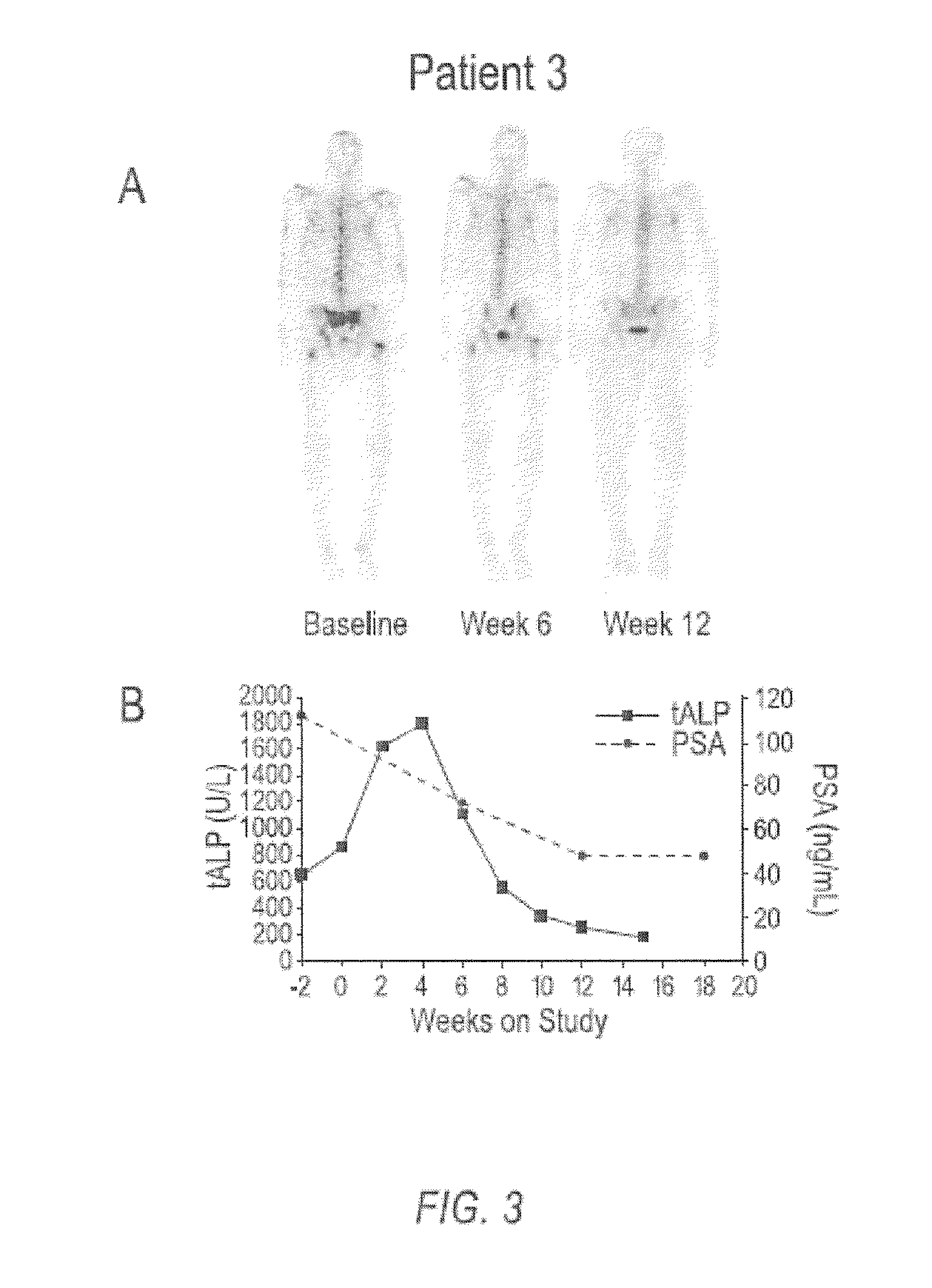
case study 1
[0136]Compound 1 is an orally bioavailable multitargeted tyrosine kinase inhibitor with potent activity against MET and VEGFR2. Compound 1 suppresses MET and VEGFR2 signaling, rapidly induces apoptosis of endothelial cells and tumor cells, and causes tumor regression in xenograft tumor models. Compound 1 also significantly reduces tumor invasiveness and metastasis and substantially improves overall survival in a murine pancreatic neuroendocrine tumor model. In a phase 1 clinical study, Compound 1 was generally well-tolerated, with fatigue, diarrhea, anorexia, rash, and palmar-plantar erythrodysesthesia being the most commonly observed adverse events.
[0137]Based on target rationale and observed antitumor activity in clinical studies, an adaptive phase 2 trial was undertaken in multiple indications including CRPC (http: / / clinicaltrials.gov / ct2 / results?term=NCT00940225 for Study NCT00940225 last visited Sep. 20, 2011)), in which Compound 1 was administered as a 100 mg dose to patients....
case study 2
[0149]In a phase 2 adaptive randomized discontinuation trial (RDT), Compound 1 resulted in resolution or stabilization of metastatic bone lesions on bone scan in 82 of 108 (76 percent) patients evaluable by this method. The majority of patients treated with Compound 1 reported reduced bone pain and reduced reliance upon narcotic pain medication. A total of 83 patients had bone metastases and bone pain reported at baseline, and at least one post-baseline assessment of pain status. Of these patients, 56 (68%) had pain improvement at either Week 6 or 12. Narcotic analgesic medication was required at baseline for control of bone pain in 67 patients assessable for post-baseline review of narcotic consumption. Of these 67 patients, 47 (70%) were able to decrease or discontinue narcotic medication for bone pain. Data on bone pain and narcotic use, as assessed by the investigator, were collected retrospectively. These results suggest that Compound 1 can be used to treat and ameliorate bone ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com