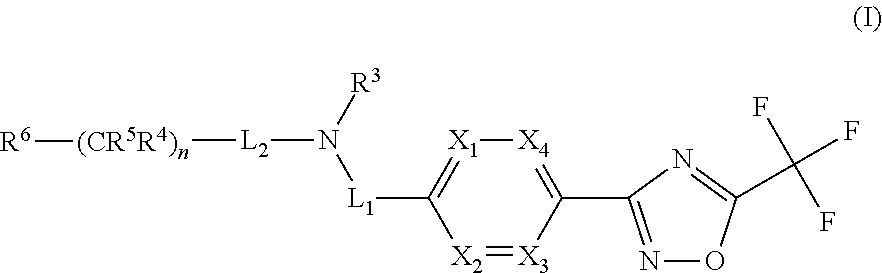
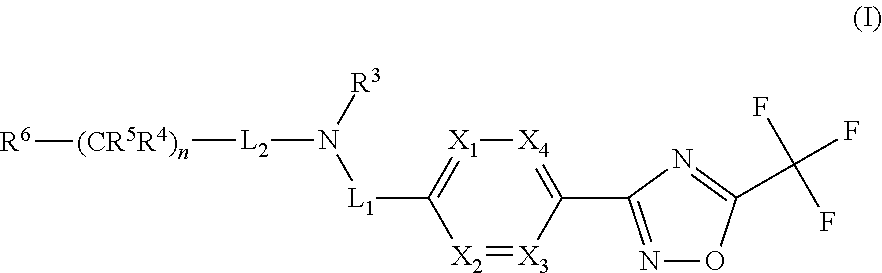
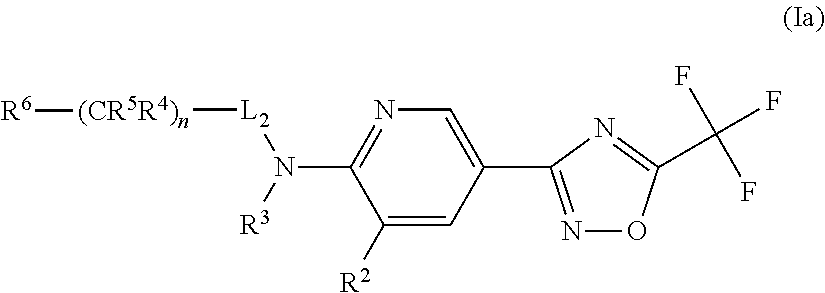
Novel trifluoromethyl-oxadiazole derivatives and their use in the treatment of disease
a trifluoromethyloxadiazole and derivative technology, applied in the field of new trifluoromethyloxadiazole derivatives, can solve problems such as interference with the cell transcriptional machinery, and achieve the effects of reducing or inhibiting, reducing symptoms, and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
N-(5-(5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl)pyrazin-2-yl)acetamide
[0315]
5-Amino-N′-hydroxypyrazine-2-carboximidamide
[0316]To 2-amino-5-cyanopyrazine (Ark Pharm Inc) (4.87 g, 40.5 mmol) and hydroxylamine hydrochloride (6.20 g, 89 mmol) in EtOH (30 mL) was added triethylamine (9.44 g, 93 mmol). The reaction was stirred at 80° C. for 1 h. The precipitate was filtered, washed with a small volume ethanol and dried on high vacuum to give 6-amino-N′-hydroxypyrazine-2-carboximidamide (5.9 g, 38.5 mmol, 95% yield) as a yellow powder. HPLC RT=0.593 min (Method A), ESIMS [M+H]+=154
5-(5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl)pyrazin-2-amine
[0317]To 5-amino-N′-hydroxypyrazine-2-carboximidamide (1.12 g, 7.31 mmol) in dry THF (6 mL) at rt was added 2,2,2-trifluoroacetic anhydride (4.61 g, 21.94 mmol). The dark-yellow solution was then heated up and stirred at reflux temperature for 16 h. Subsequently the reaction was quenched by addition of a 25 mol % ammonia solution to reach a basic pH. Brine...
example 2
4-Cyano-N-(5-(5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl)pyrazin-2-yl)benzamide
[0319]
[0320]4-Cyano-N-(5-(5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl)pyrazin-2-yl)benzamide was made using a process analogous to that described in Example 1 HPLC RT=3.577 min (Method A), ESIMS [M+H]+=361, light pink powder
[0321]1H NMR (400 MHz, DMSO-d6) δ ppm 11.92-11.95 (m, 1H) 9.64-9.66 (m, 1H) 9.19-9.22 (m, 1H) 8.21-8.24 (m, 1H) 8.19-8.22 (m, 1H) 8.07-8.09 (m, 1H) 8.05-8.07 (m, 1H)
example 3
N-methyl-N-(pyridin-4-ylmethyl)-5-(5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl)pyrimidin-2-amine
[0322]
2-(methyl(pyridin-4-ylmethyl)amino)pyrimidine-5-carbonitrile
[0323]To a solution of 2-(methylthio)pyrimidine-5-carbonitrile (Biofine International Inc.) (390 mg, 2.58 mmol) in 1,4-dioxane (3 mL), N-methyl-1-(pyridin-4-yl)methanamine (Fisher Scientific International—Maybridge) (789 mg, 2.50 mmol) was added at rt. The resulting reaction mixture was heated in the microwave oven at 170° C. for 10 h. Subsequently the solvent was evaporated at high vacuum and the remaining oily residue subjected to purification by flash chromatography (ISCO CombiFlash Rf; 80 g silicagel, dichlormethane / methanol) to give 2-(methyl(pyridin-4-ylmethyl)amino)pyrimidine-5-carbonitrile (404 mg, 1.65 mmol, 64% yield) as yellow powder. HPLC RT 1.323 min (Method A); ESIMS [M+1]+ 226
N′-hydroxy-2-(methyl(pyridin-4-ylmethyl)amino)pyrimidine-5-carboximidamide
[0324]To a mixture of 2-(methyl(pyridin-4-ylmethyl)amino)pyrimid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| physical parameter | aaaaa | aaaaa |
| optically active | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com