Human monoclonal antibodies broadly protective against influenza b virus and methods of using the same
a technology of monoclonal antibodies and influenza b, applied in the field of influenza b viral infection treatment materials and methods, can solve the problems of limited efficacy and continuous public health threat, and achieve the effect of showing therapeutic efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0115]Human monoclonal antibodies (HuMAbs) were prepared in accordance with the procedure described in Kubota-Koketsu et al., Biochemical and Biophysical Research Communications 387:180-185 (2009). Healthy volunteers were vaccinated with the HA split vaccine including A / Brisbane / 59 / 2007 (H1N1), A / Uruguay / 716 / 2007 (H3N2), and B / Florida / 4 / 2006 strains. One to two weeks later, the vaccine-derived PBMCs were fused with SPYMEG cells and after screening and cloning, three hybridoma clones producing HuMAbs, designated 5A7, 3A2, and 10C4, were established. The reactivity of the HuMAbs was tested by IFA and Western blotting.
[0116]Briefly, 10 ml blood was drawn from a healthy volunteer vaccinated in the 2008 / 2009 winter season with trivalent HA split vaccine, which included A / Brisbane / 59 / 2007, A / Uruguay / 716 / 2007, and B / Florida / 4 / 2006 (The Research Foundation for Microbial Diseases of Osaka University, Kagawa, Japan), and then the PBMCs were collected by density gradient centrifugation through...
example 2
[0125]To determine the epitope regions of B / Florida / 4 / 2006 recognized by the three HuMAbs, escape mutants were selected. The escape mutants were selected by the incubation of B / Florida / 4 / 2006 with HuMAbs. Escape mutants were selected by culturing B / Florida / 4 / 2006 in the presence of MAb as described previously (Gulati et al., Journal of Virology 76:12274-12280), with minor modification. Viruses were incubated with MAb serially diluted 10-fold (to give final concentrations of 0.0025 to 2.5 mcg / ml) at 37 C. for 1 hour. Then MDCK cells were inoculated with the mixtures and cultured in DMEM / F-12+GlutaMAX™-I supplemented with 0.4% BSA, antibiotics, and 2 mcg / ml acetylated trypsin. After culturing for 72 hours, the supernatants were collected and subjected to VN and HI assays. Those viral samples that showed a reduced VN50 and HI titer were subjected to direct sequencing analysis of the entire HA gene.
[0126]Direct sequencing analysis was performed as follows. Viral RNA extracted with QIAam...
example 3
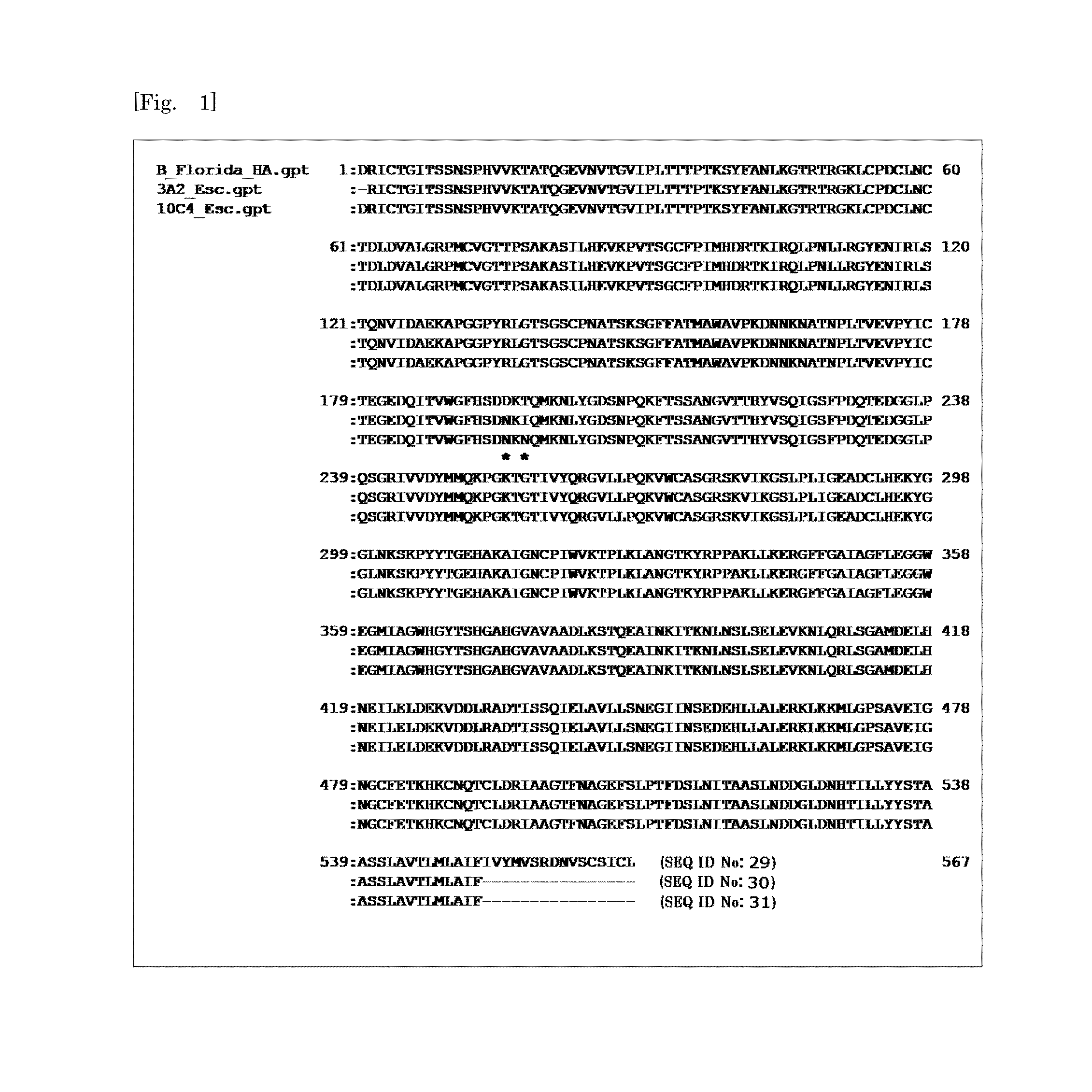
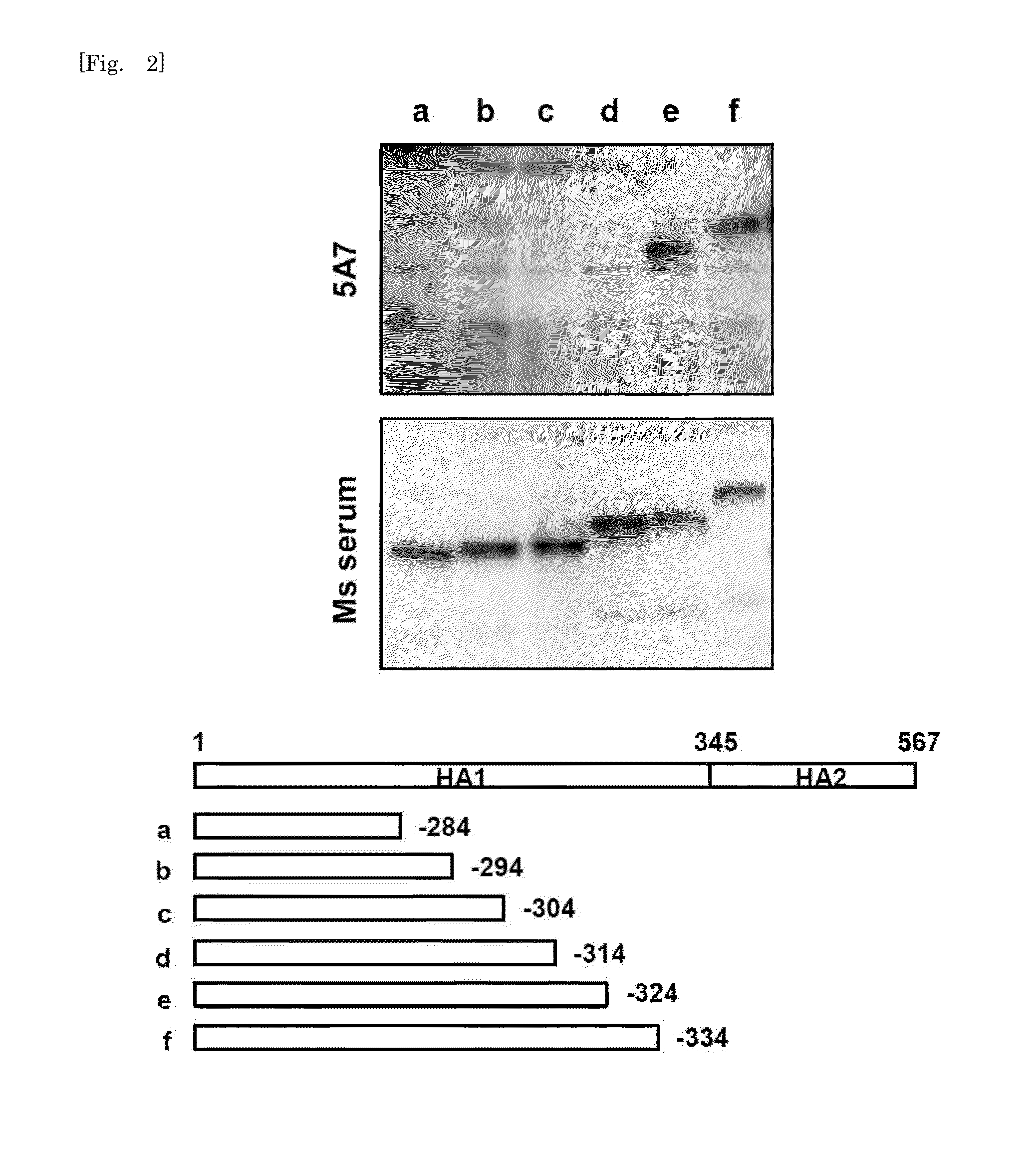
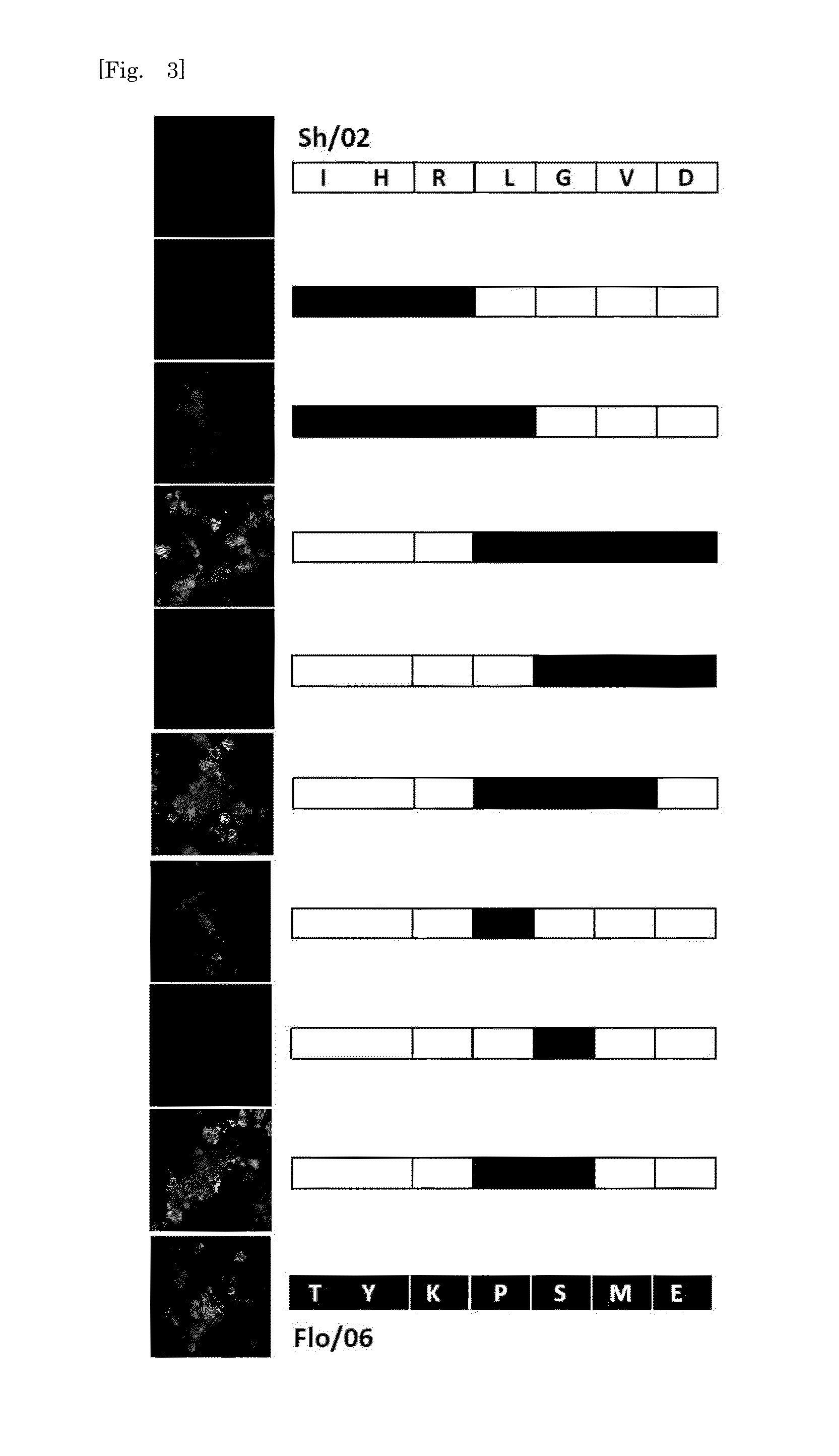
[0129]HuMAb 3A2 showed low reactivity against B / Shanghai / 361 / 2002 and was therefore examined for an additional distinct epitope region. To do this, various chimeric sequences of HA were constructed from B / Florida / 4 / 2006 and B / Shanghai / 361 / 2002, which differ at seven residues (positions 37, 40, 88, 131, 227, 249, 456), expressed in plasmids, and transfected into 293T cells. IFA of the chimeric HA proteins expressed in 293T cells showed that 131P and 227S were essential for the reaction with 3A2 (FIG. 3). These results indicate that the epitope of 3A2 is dependent on residues at positions 131, 194, 196, and 227, and the epitope of 10C4 is dependent on residues at positions 194 and 196. The epitope regions to which the three HuMAbs map are shown in a HA trimer three-dimensional model in FIG. 4. HuMAbs 3A2 and 10C4 recognized the top of the globular head including the 190-helix antigenic site, whereas 5A7 reacted with the stalk region distant from the viral membrane.
[0130]HuMAb, 5A7 rec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com