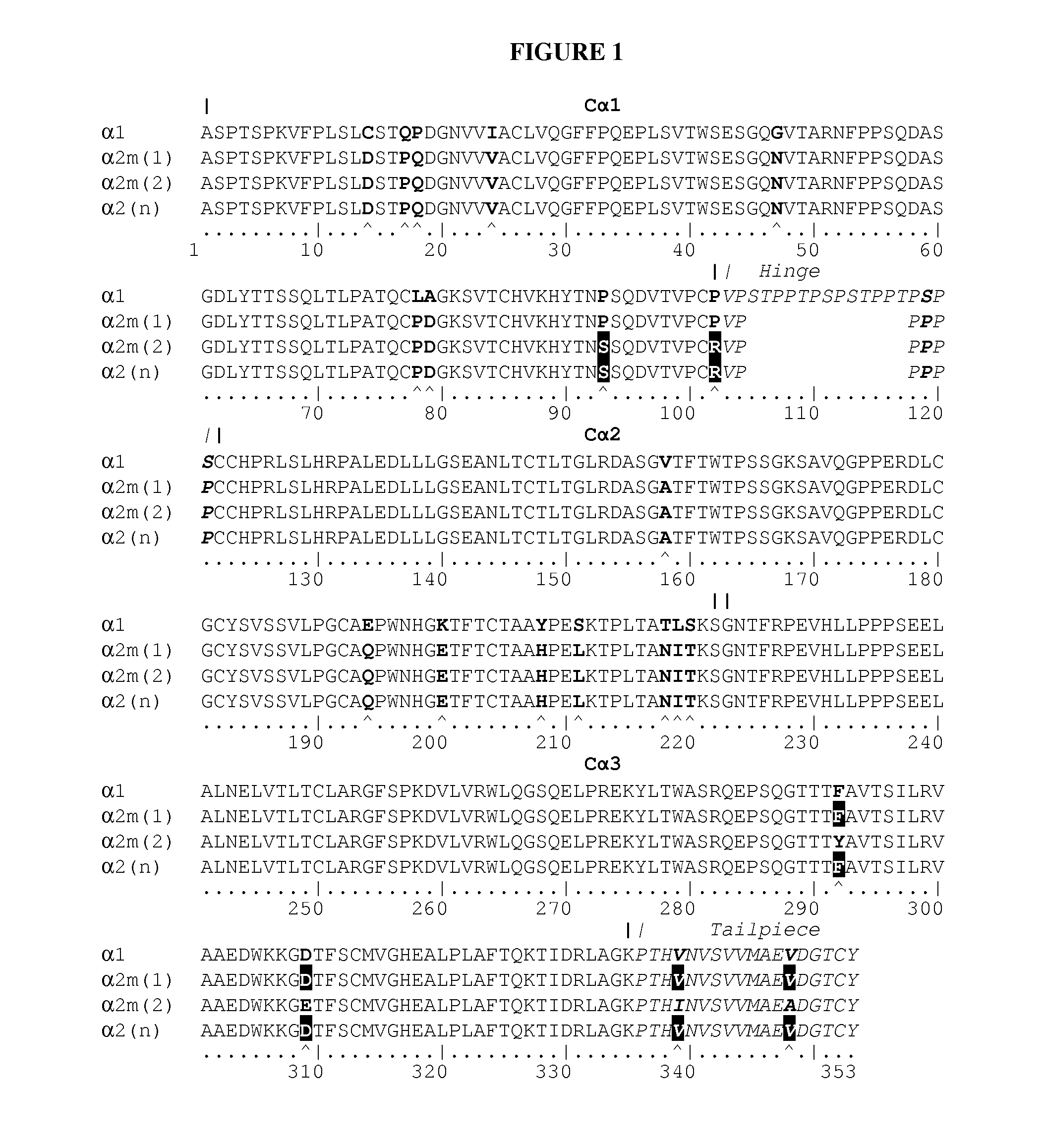Compounds and methods for treating inflammatory diseases
a technology of iga antibodies and compounded igas, which is applied in the field of monoclonal secretory iga antibodies, can solve the problems of difficult undesirable use of animal-derived antibodies and polyclonal antibodies, and the inability to purify several other chromatographic media. the potential of purification of carorx antibodies is small
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Stable Expression of Anti-IL-12 / 23 Secretory Protein Based on Ustekinumab (UKB-SA1) in Lemna
[0126]a) Construction of Vectors
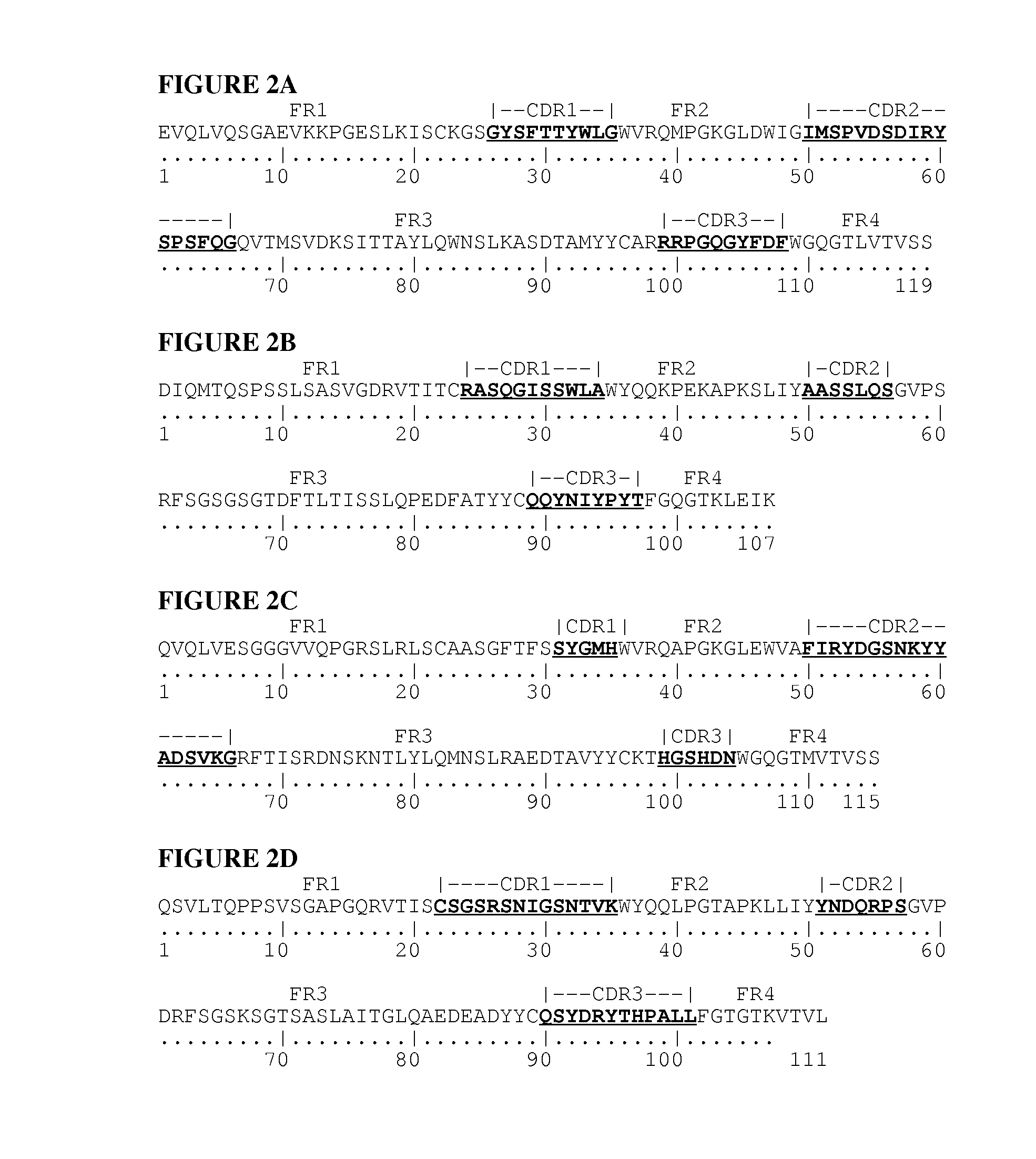
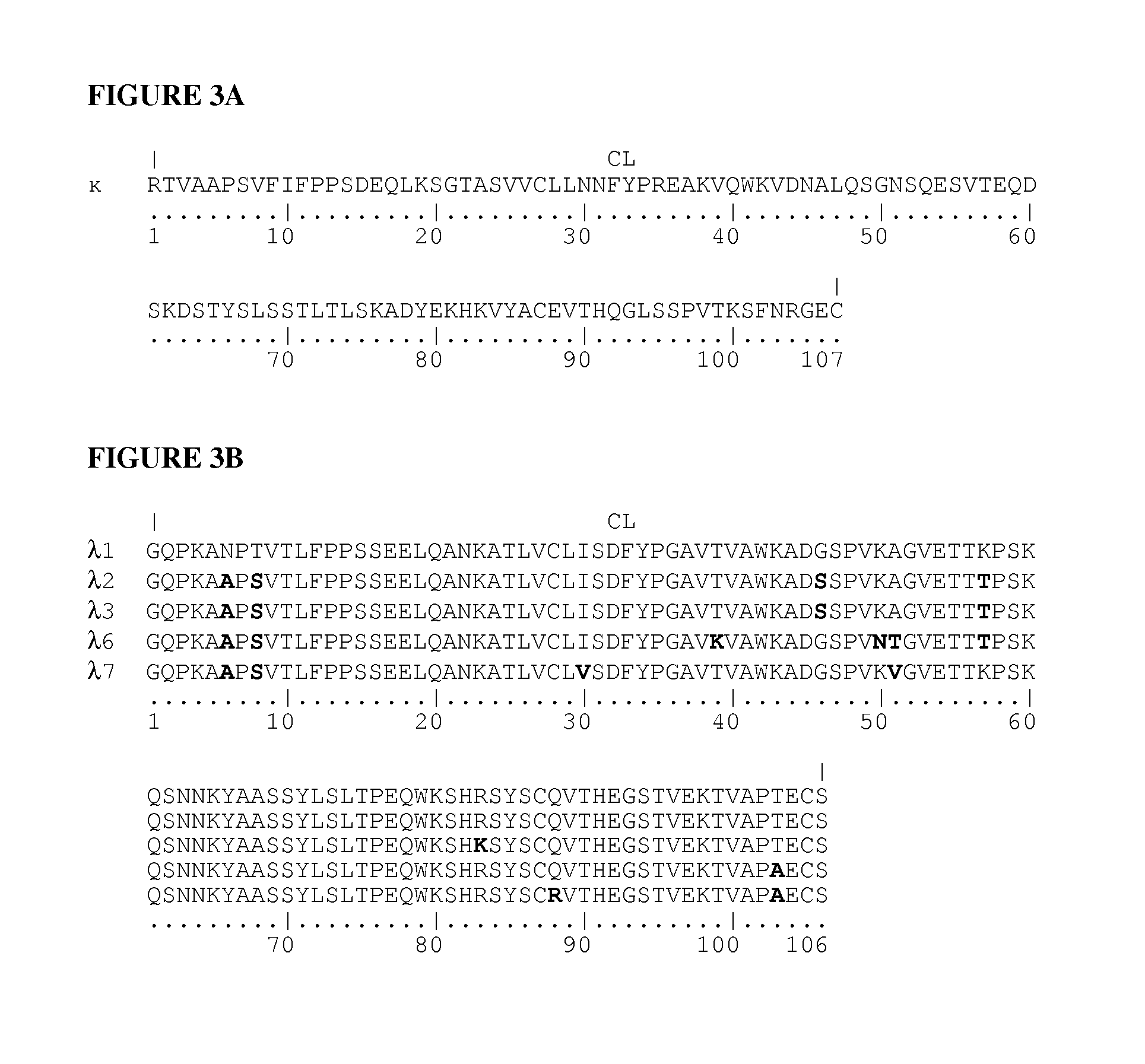
[0127]Synthetic genes were designed for each of the 4 different protein chains of an anti-p40 (anti-IL-12 / 23) secretory IgA. The amino acid sequence of the heavy chain consisted of the rice α-amylase secretion signal (SEQ ID NO:17) joined to the N-terminal amino acid of the variable part of the heavy chain of anti-IL12 / 23 IgG1 antibody Ustekinumab (Stelara®, CAS number 815610-63-0, SEQ ID NO:1) which in turn is joined to the N-terminal amino acid of the constant part of a human IgA1 heavy chain (SEQ ID NO:5). The amino acid sequence of the light chain consisted of the rice α-amylase secretion signal (SEQ ID NO:17) joined to the N-terminal amino acid of the light chain sequence of ustekinumab (CAS number 815610-63-0), which combines an anti-p40 (anti-IL-12 / 23) binding variable part (SEQ ID NO:2) with a human κ-light chain constant part (SEQ ID NO:9). The SC-cha...
example 2
Isolation and Purification of UKB-SA1 and UKB-SA1g0 Secretory IgA Antibody from Lemna
[0136]Biomass from transgenic Lemna expressing UKB-SA1 or UKB-SA1g0, having variable regions that are the amino acid sequence of the variable regions (antigen binding regions) of ustekinumab, was homogenized in 50 mM Sodium phosphate, 0.3M Sodium chloride, buffer pH 7.4, at a buffer to tissue ratio of 4:1. An acid precipitation step was performed on the crude extract to remove the enzyme ribulose bis-phosphate carboxylase (RuBisCo) and other plant proteins by adjusting the extract to pH 4.5 using 1M Sodium acetate, pH 2.5. The precipitate was removed by centrifugation of the material at 14,000×g for 30 minutes at 4° C. The supernatant was adjusted to pH 7.4 and filtered to 0.22 μm prior to IMAC chromatography.
[0137]IMAC purification: A chelating Sepharose FF (GE Healthcare prod. Nr. 17-0575-01) column was prepared according to manufacturer instructions (28-4047-39 AC). The column was charged with 3...
example 3
Binding of UKB-SA1 and UKB-SA1g0 to IL12
[0146]The binding of purified anti-IL-12 / 23 SIgA, with variable regions taken from ustekinumab and produced in Lemna as in Examples 1 and 2, to IL-12 was determined. The binding of both UKB-SA1 and UKB-SA1g0 products were determined in comparison to ustekinumab (STELARA®) and colostral SIgA. Plates were coated with IL-12 (Abcam, AB52086) 1 μg / ml. Detection of bound UKB-SA1 / UKB-SA1g0 (secretory IgA) and ustekinumab (IgG1) antibodies was performed using a 1:1500 fold dilution of anti human kappa chain antibody (Abbiotec, cat. no. 250987), 100 μl per well, for one hour at RT, washing 3 times, 30 seconds with 200 μl PBS / 0.05% Tween with shaking, followed by incubation with a 1:1500 fold dilution of donkey anti mouse HRP conjugated (Emelca biosciences, MS3001), 100 μl per well, for one hour at RT.
[0147]The UKB-SA1, UKB-SA1g0 and ustekinumab antibodies all bound with high affinity to IL-12 under the conditions of this assay. For UKB-SA1 and UKB-SA1g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| w/w | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com