Method for chromatography reuse
a chromatography and reuse technology, applied in the field of chromatography, can solve the problems of protein carryover, protein cost increase, and protein cost increas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Protein Carryover
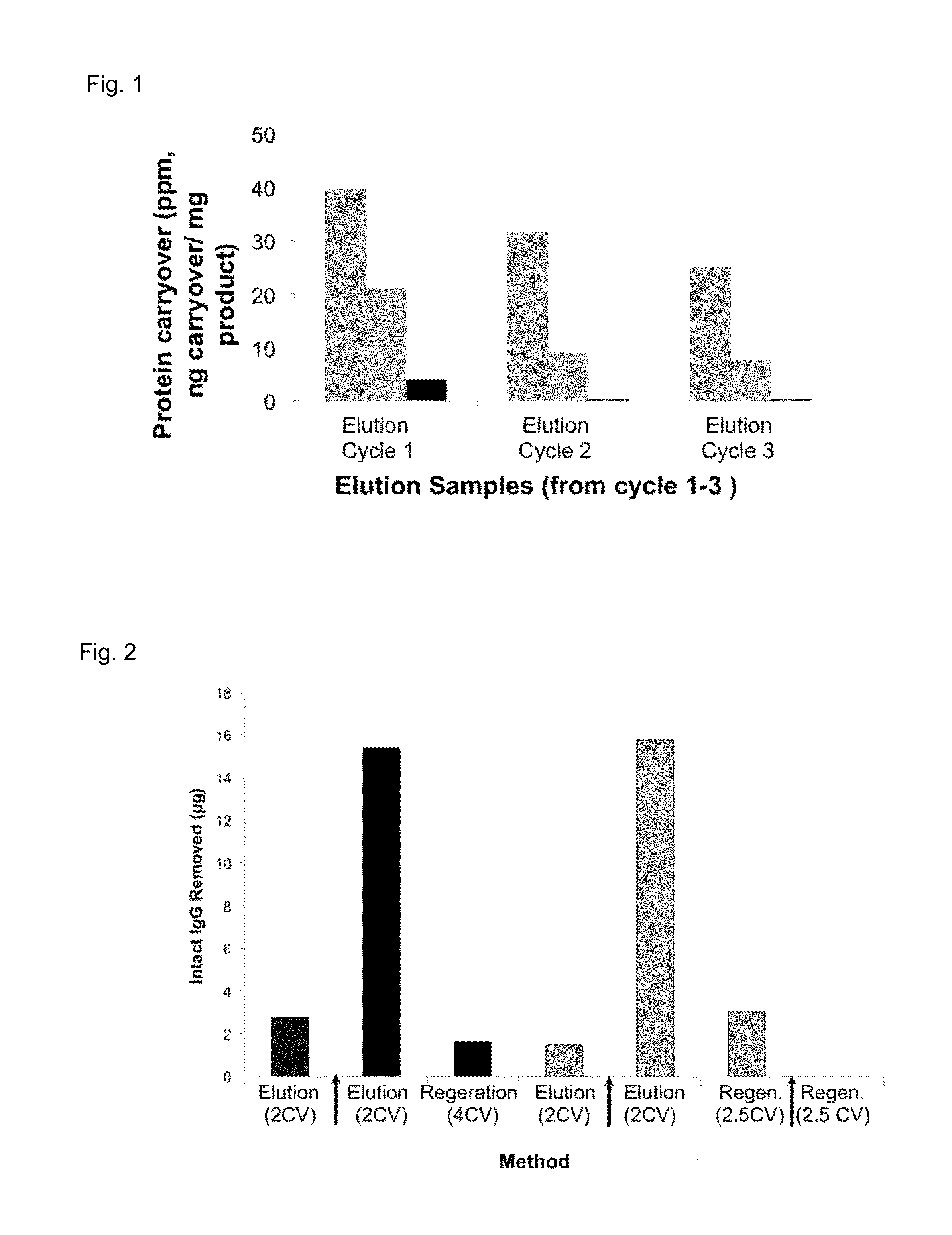
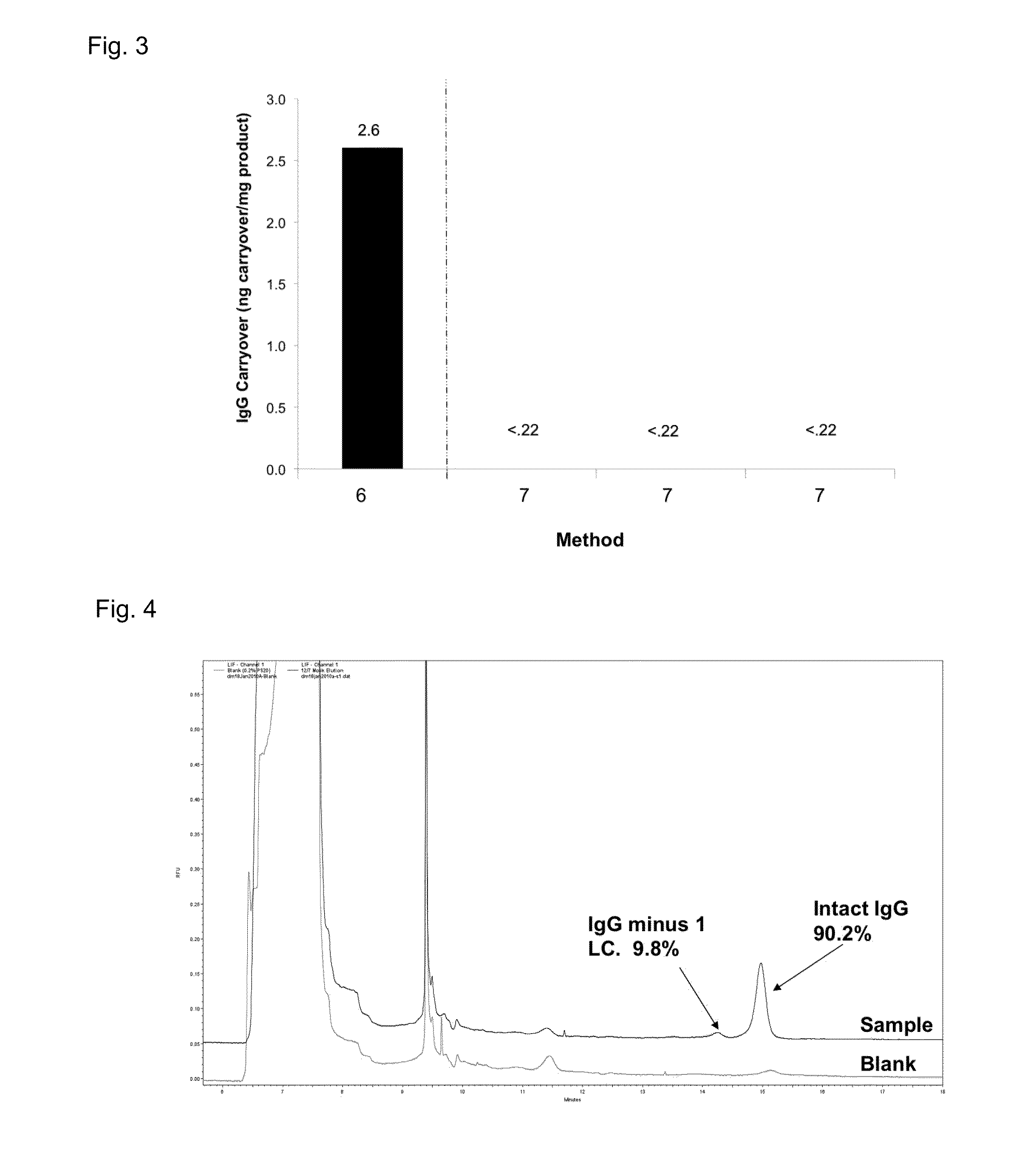
[0301]This example describes an effort to quantify protein carryover from sample to sample a pre-cleaning test run was performed on lab scale using a standard Protein A affinity material (0.66×20 cm). This run was called a “mock run” as the process was run according to a standard purification procedure except the load cycle was simulated with the equilibration buffer. The elution pool was collected, as per a typical Protein A process, and analyzed to determine the presence of protein. The analysis revealed that 20-30 ppm of protein carryover was present in the “mock elution” in the absence of any additional column cleaning. The result was confirmed with a second run.
[0302]In order to determine safe carryover levels, a risk assessment was conducted to determine acceptable immunoglobulins (IgG) and protein carryover levels in mAbs, and a substance-specific Acceptable Daily Exposure (ADE) for IgG was calculated. A comparison of the ADE to EDI, resulted in a “worst case...
example 2
Evaluation of Ion Exchange Columns for Multi-Product Use
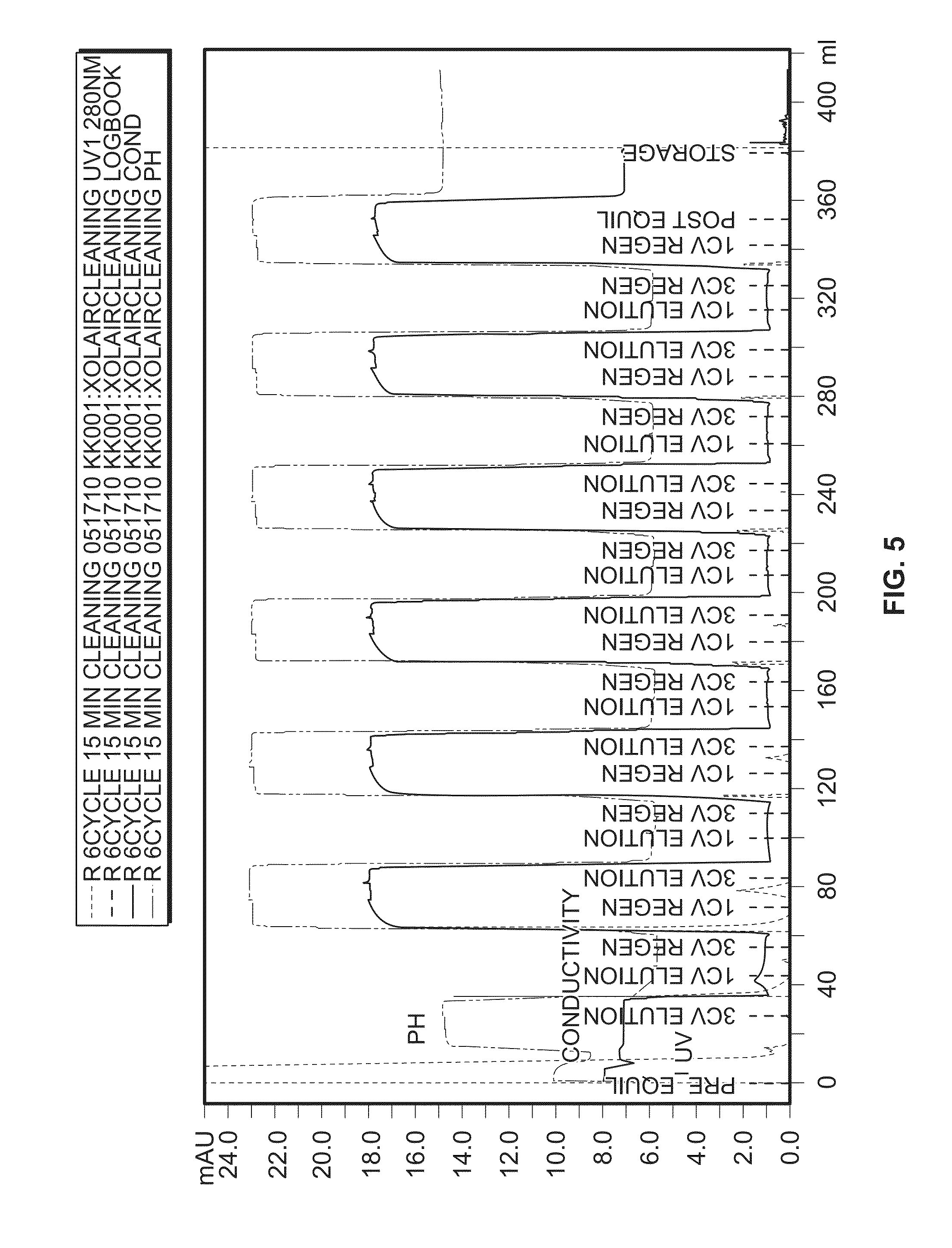
[0333]Studies were performed to determine if a similar cleaning process could be developed for ion exchange chromatographies. MAbA and MAbB from ProA pools were loaded onto cation exchange columns (POROS) or anion exchange columns (QSFF). Following normal elution, the columns were then subject to a “mock elution”. Fractions were analyzed for the presence of MAbA and / or MabB using a MabSelectSure assay, limit of detection was 0.82 ng / mL. As seen in FIG. 13, mock elution results indicated the need for additional cleaning of the columns
[0334]The following clean-in-place (CIP) procedure was tested.
I.3 CV Equilibration BufferII.2 CV 0.5N NaOH10 minute static holdIII.1 CV 0.5N NaOH10 minute static holdIV.1 CV 0.5N NaOHV.Post-cleaning mock run
[0335]Samples were conditioned with low concentration of detergent (0.1% polysorbate 20, 0.05% sodium azide) to prevent sample from sticking to the wall of the container. Samples were adjusted to...
example 3
Evaluation of ProSep a Column for Multi-Product Use
[0339]Different cleaning solutions were evaluated at small scale in order to assess which solutions were most effective at reducing product carryover. Several different categories of solutions were tested, including acids, chaotropes, salts, and organic solvents. This study was designed to follow the standard protein A antibody process in order to best mimic generic process conditions, although actual processing conditions may differ depending on the specific product used.
[0340]Flow was directed in a downflow direction through the column for all processes with the exception of the cleaning cycle. Throughout the cleaning cycle, flow was directed upwards through the column in hopes of creating a best case cleaning scenario. Since feedstock is directed through the column in a downward direction during the loading cycle, the top of the Protein A column would theoretically foul the most. By directing flow of the cleaning cycle upwards, t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Force | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com