Anti-dr5 family antibodies, bispecific or multivalent Anti-dr5 family antibodies and methods of use thereof
a family of anti-dr5 family antibodies and antibody technology, applied in the field of immunology and oncology, can solve the problems of short half-life drawback, achieve stronger agonistic action, induce dr5 apoptosis, and induce apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Murine MAb Anti-DR5
[0287]This example illustrates the preparation of hybridoma cell lines secreting anti-DR5 antibodies.
[0288]Antibodies.
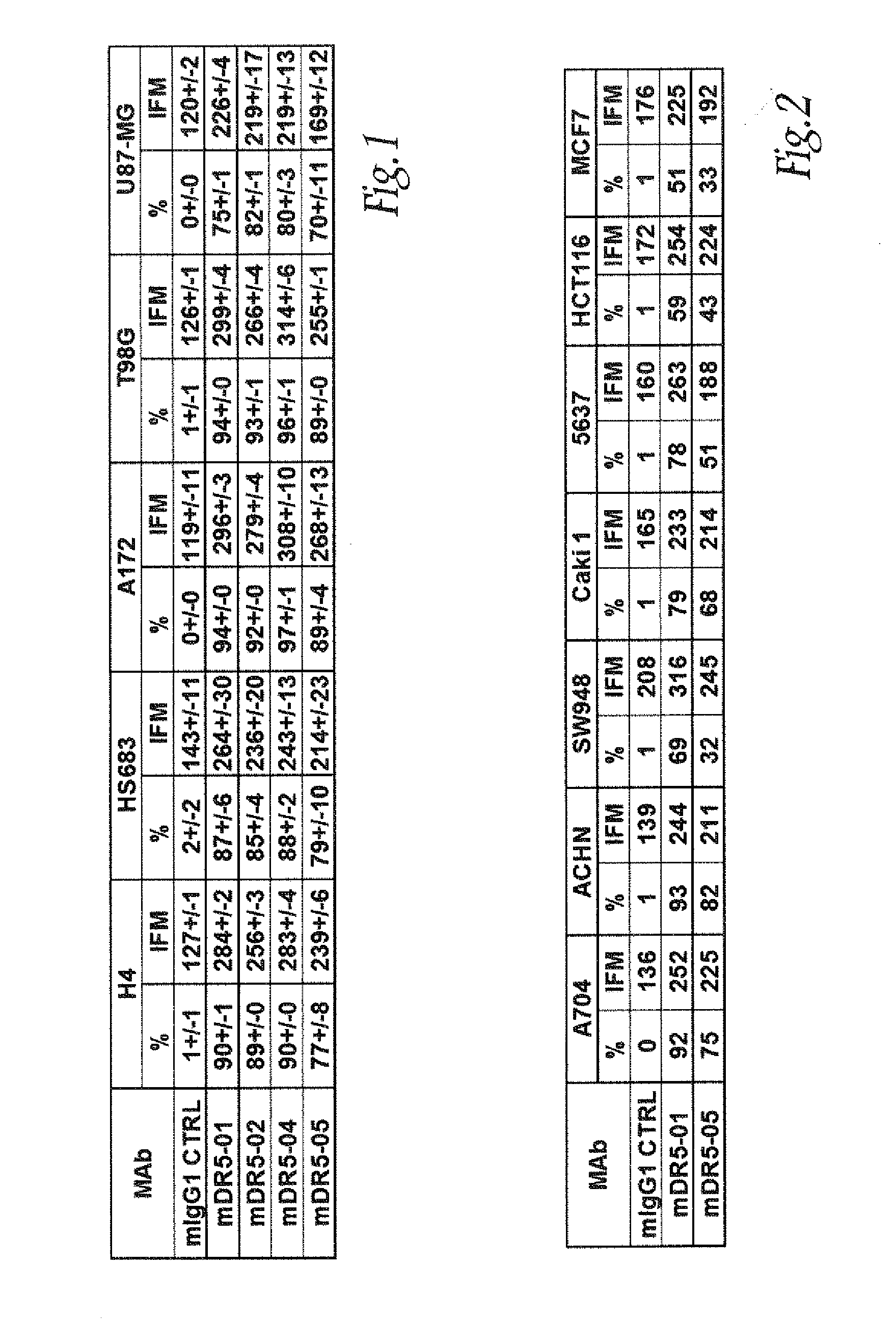
[0289]The anti-DR5 antibodies, murine monoclonal antibodies specific for DR5 were produced using standard hybridoma techniques (Zola et al., Aust J. Exp Biol Med Sci. 1981; 59:303-6). Briefly, mice were given i.p. injections of recombinant DR5 (10 μg), (R&D Systems, Lille, France) on weeks 0, 2 and 4. This was followed by an i.v. injection of recombinant DR5 (10 μg) and the splenocytes were fused with mouse myeloma line X63-Ag8.653. Hybridoma supernatants were screened for DR5 binding by ELISA and by flow cytomery on DR5 positive cell lines. A murine MAb panel anti-DR5 noted mDR5-01, mDR5-02, mDR5-04 and mDR5-05 were obtained.
example 2
Cell Culture
[0290]Various tumor-derived cell lines are among the target cells that may be contacted with TRAIL, anti-DR5 MAb alone, MAb combination, in such assay procedures.
[0291]Cell lines. The established human neuroglioma cells H4, HS683 or A172 (available from ATCC) and the established human lung adenocarcinoma cells A549 were grown in Dulbecco's Modified Eagle's Medium (Sigma, St Quentin Fallavier, France) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Sigma, St Quentin Fallavier, France), 4 nM L-glutamine (Sigma, St Quentin Fallavier, France) and 100 U / mL, 100 μg / mL penicillin-streptomycine (Sigma, St Quentin Fallavier, France). The established human glioblastoma astrocytoma cells U87MG or T98G, the human kidney adenocarcinoma cells A704, the human kidney adenocarcinoma cells ACHN and the human breast adenocarcinoma cells MCF7 (available from ATCC) were grown in Eagle's Minimum Essential Medium (Sigma, St Quentin Fallavier, France) supplemented with 10% heat...
example 3
In Vitro Biologic MAb Activity
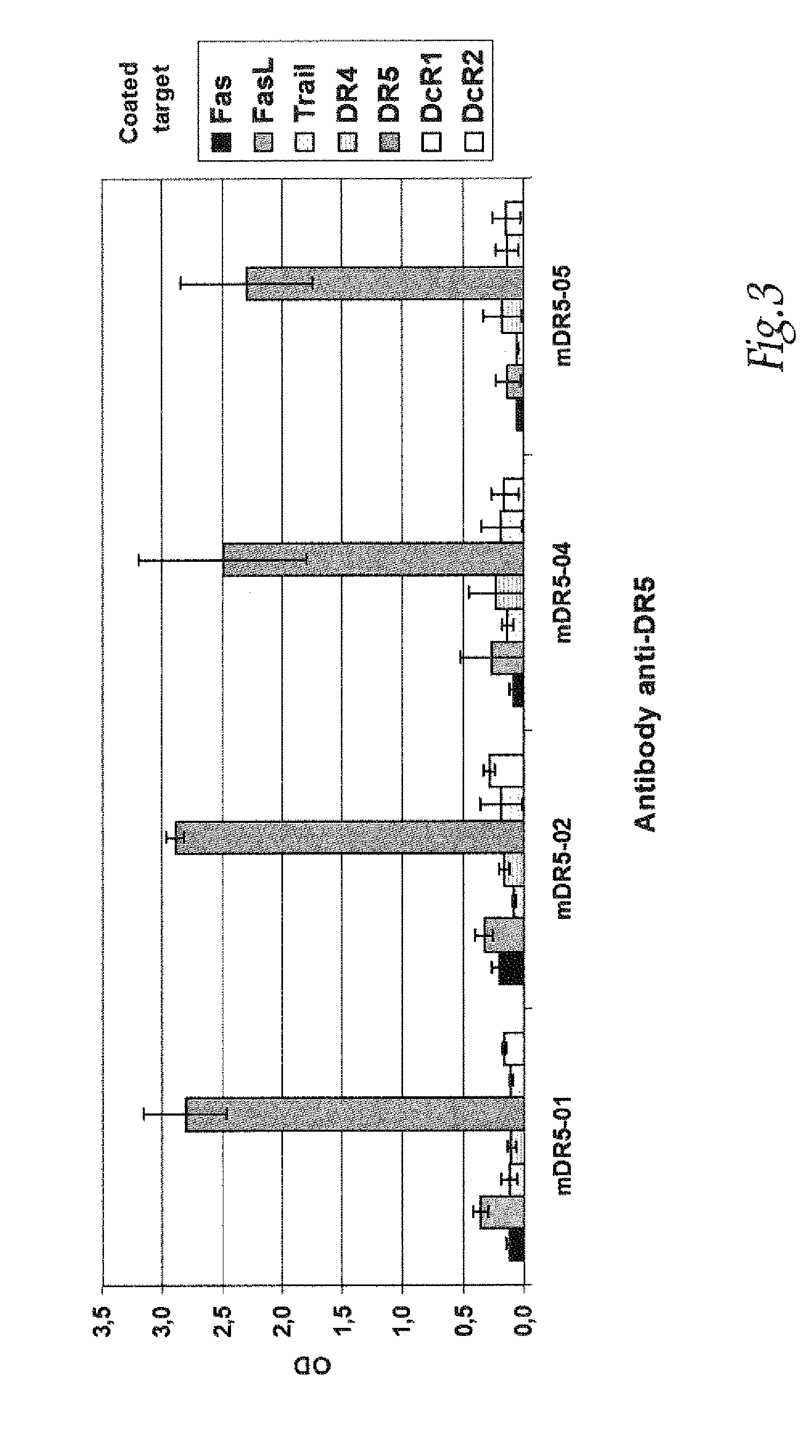
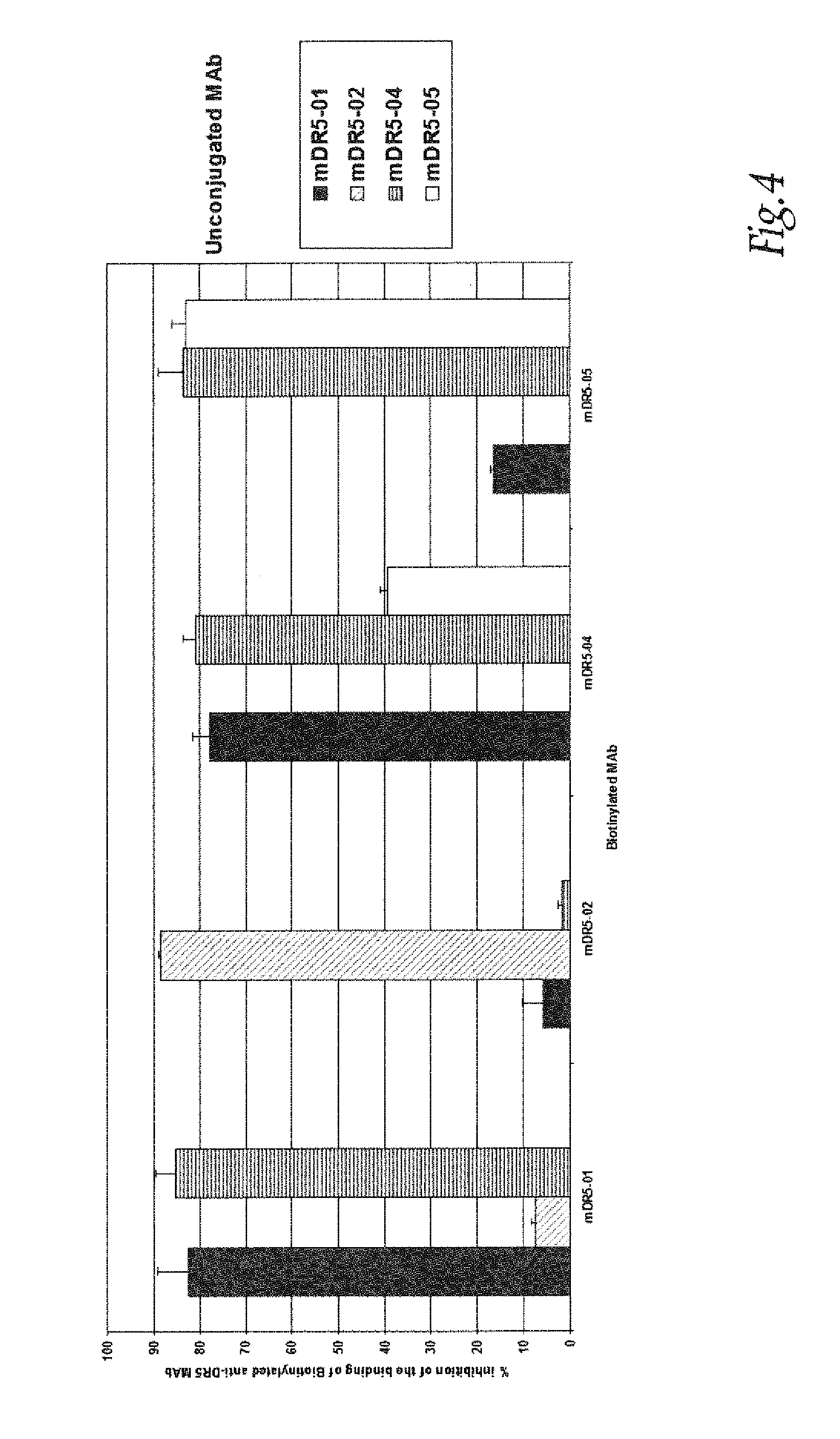
[0300]This example illustrates methods of evaluating the anti-DR5 MAb impact on TRAIL cellular binding on their ability to trigger cellular cytotoxic effect on cancer cells. These components may be assayed for anti-tumour activity, using any of a number of suitable assays, including but not limited to assays for the ability to slow tumour growth or to kill cancer cells in vitro. Various tumour-derived cell lines are among the target cells that may be contacted with MAb combination, in such assay procedures.
[0301]To identify or select anti-DR5 antibody combination which induce apoptosis, loss of membrane integrity as indicated by, e.g. PI is assessed relative to control (untreated cells) and compared to recombinant TRAIL (FIG. 8). The ability to slow tumour growth is assessed by ATP or BrDU quantification (FIG. 6, FIG. 7). The apoptotic response is assessed by quantification of cleaved caspase 3 (FIG. 9) or cleaved Poly-(ADP-Ribose)-Polymerase (PARP), (F...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com