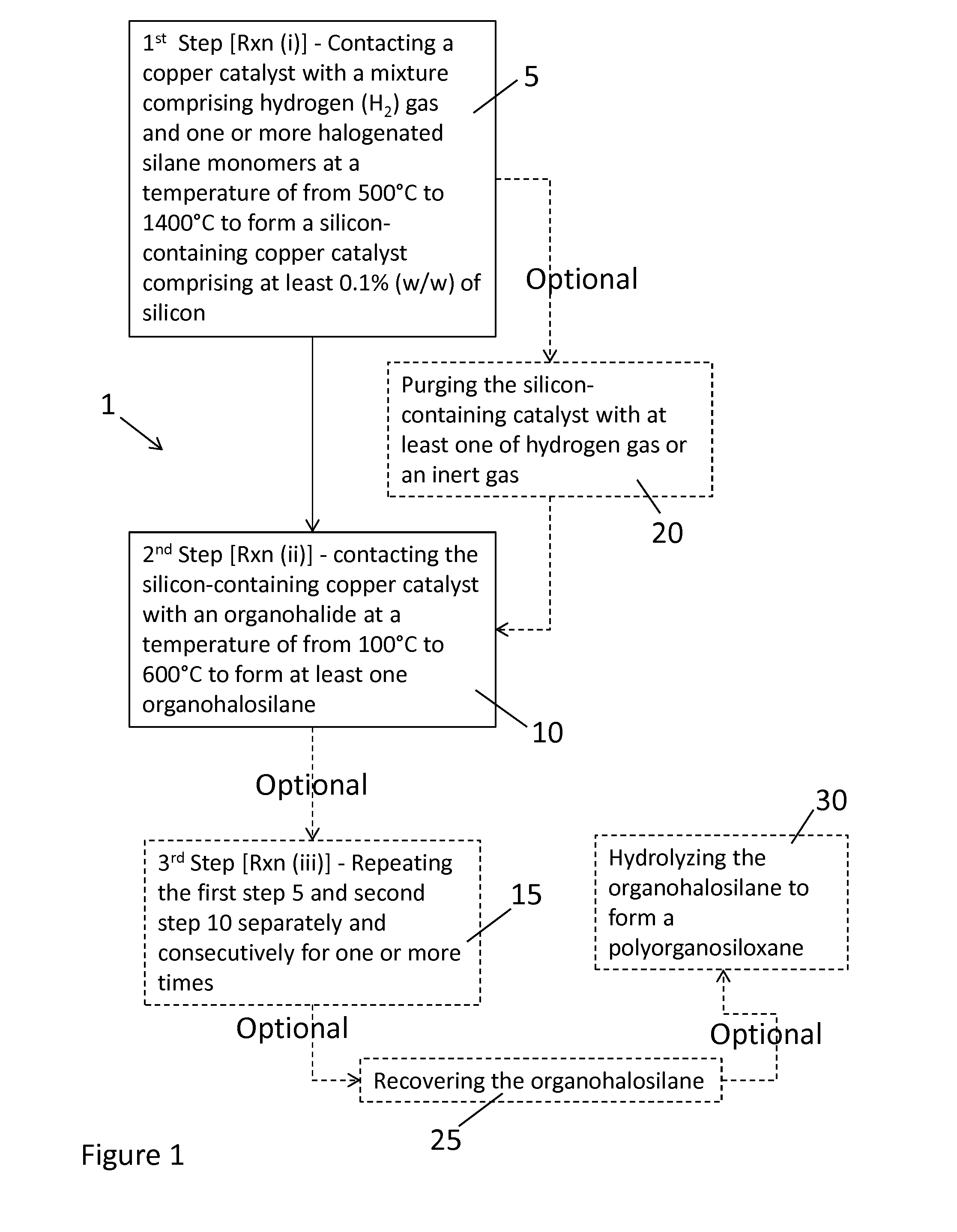
Method Of Preparing An Organohalosilane
a technology of organohalosilane and halide, which is applied in the field of organohalosilane preparation, can solve the problems of large amounts of metal halide by-products that require costly disposal, and achieve the effects of less energy, better selectivity and more economical us
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Methylchlorosilanes Using Copper Catalyst Treated at 750° C. with H2 / SiCl4 at Different Ratios of H2:SiCl4
[0046]A copper catalyst (0.5 grams) comprising an activated carbon supported mixture of copper, gold and magnesium, prepared by incipient wetness impregnation, is treated with H2 / SiCl4 for 30 min at 750° C.-950° C. by bubbling H2 through a stainless steel SiCl4 bubbler. The total flow of H2 and SiCl4 is 150 sccm and the mole ratio of H2 to SiCl4 is varied from 4:1 to 1:1. The SiCl4 flow is controlled by H2 flow by varying the bubbler temperature between 14.6° C. to 37.2° C. The gas and vapor leaving the bubbler is fed into the glass tube of a flow reactor containing the copper catalyst to form a silicon-containing copper catalyst comprising from 24-47% (w / w) Si. After 30 minutes, the SiCl4 flow is ceased and a hydrogen flow of 100 sccm is maintained while cooling to 300° C. over a period of 1 hour. When the reactor reached 300° C., all H2 was purged from the react...
example 2
Production of Methylchlorosilanes Using Copper Catalyst Treated at 750° C. with H2 / HSiCl3
[0048]A copper catalyst (0.5 grams) comprising an activated carbon supported mixture of copper, gold and magnesium, prepared by incipient wetness impregnation, is treated in H2 / HSiCl3 for 30 min at 750° C. by bubbling H2 through a stainless steel HSiCl3 bubbler at −7.6° C. The total flow of H2 and HSiCl3 is 150 sccm and the mole ratio of H2 to HSiCl3 is 4:1. The gas and vapor leaving the bubbler is fed into the glass tube of a flow reactor containing the copper catalyst to form a silicon-containing copper catalyst up to 43% (w / w) Si. After 30 minutes, the HSiCl3 flow is ceased and a hydrogen flow of 100 sccm is maintained while cooling to 300° C. over a period of 1 hour. When the reactor reached 300° C., all H2 is purged from the reactor and catalyst with an argon flow of 50 sccm for 30 min. After 30 min, the argon flow is ceased, and CH3Cl is fed through the reactor at a flow rate of 5 sccm, 3...
example 3
Production of Methylchlorosilanes Using Copper Catalyst Treated at 750-950° C. with H2 / CH3SiCl3
[0050]A copper catalyst (0.5 grams) comprising an activated carbon supported mixture of copper, gold and magnesium, prepared by incipient wetness impregnation, is treated in H2 / CH3SiCl3 for 30 min at 750° C.-950° C. by bubbling H2 through a stainless steel CH3SiCl3 bubbler at 21.9° C. The total flow of H2 and CH3SiCl3 is 150 sccm and the mole ratio of H2 to CH3SiCl3 is 4:1. The gas and vapor leaving the bubbler is fed into the glass tube of a flow reactor containing the copper catalyst to form a silicon-containing copper catalyst up to 3% (w / w) Si. After 30 minutes, the CH3SiCl3 flow is ceased and a hydrogen flow of 100 sccm is maintained while cooling to 300° C. over a period of 1 hour. When the reactor reached 300° C., all H2 is purged from the reactor and catalyst with an argon flow of 50 sccm for 30 min. After 30 min, the argon flow is ceased, and CH3Cl is fed through the reactor at a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com