Vascular grafts and method for preserving patency of the same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Microporous Silicone Vascular Grafts as AV Shunt
Materials:
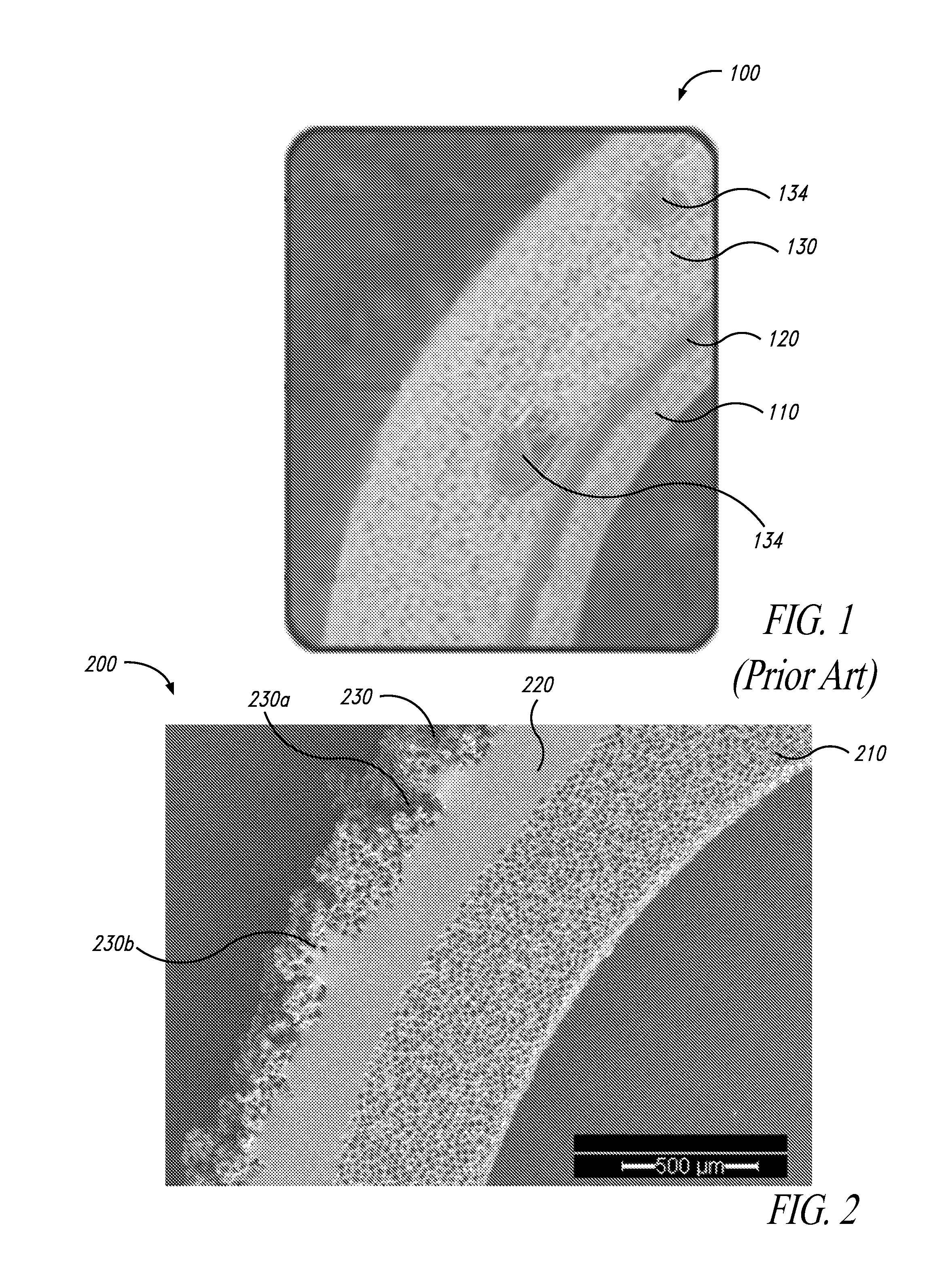
[0078]Control grafts (N=4) were ePTFE vascular grafts (from Impra) with regular wall thickness of 6 mm and spiral wrapped ribs for radial reinforcement.
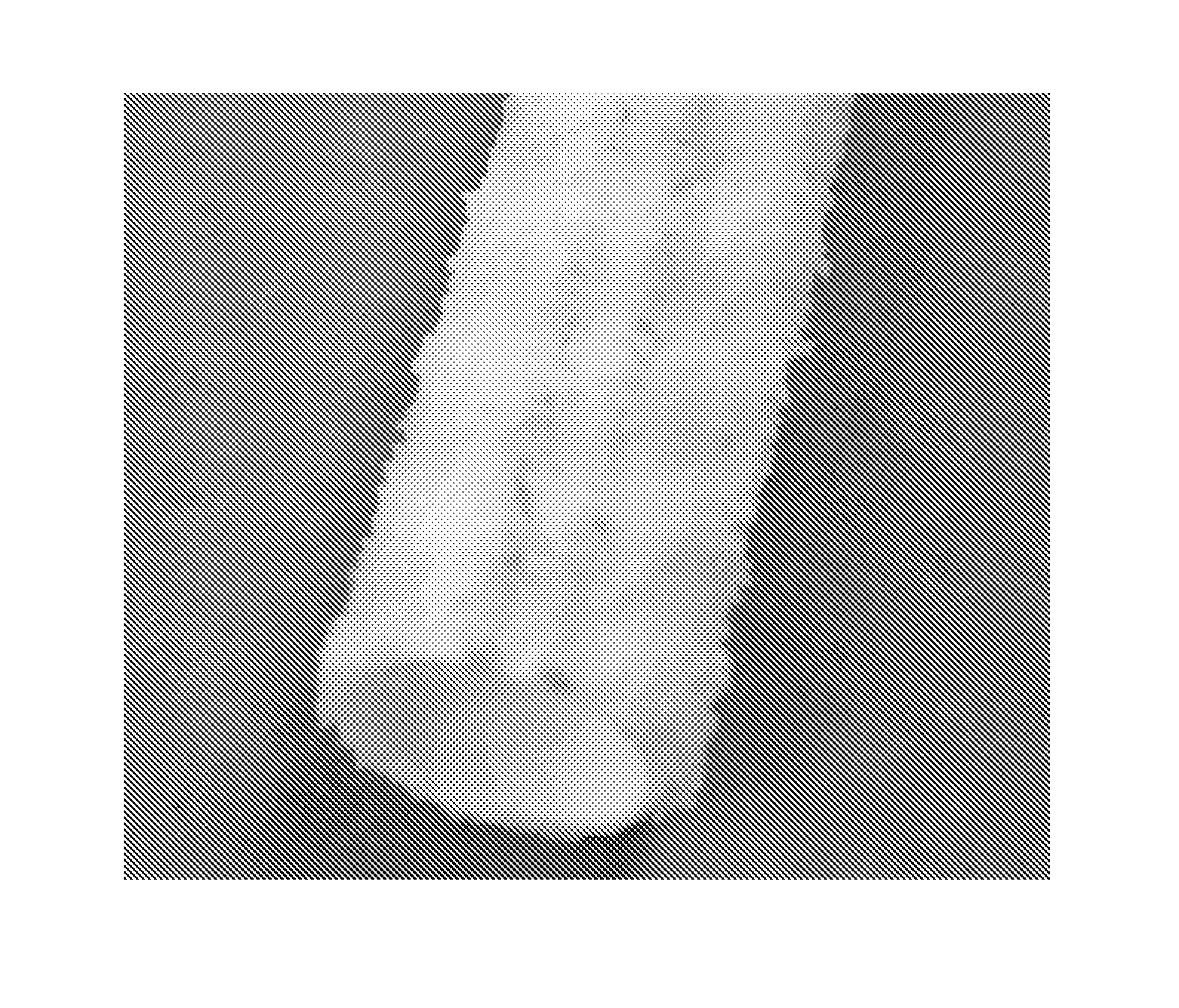
[0079]Test grafts (N=6) were 100% silicone grafts as shown in FIG. 2.
[0080]Some of the control grafts and test grafts were fitted with mid-graft percutaneous ports.
[0081]Sheep were 35-40 kg at time of implant. The animals were heparinized.
Methods
[0082]The vascular grafts were implanted in an ovine arteriovenous (AV) shunt model. The control grafts were soaked in heparinized saline prior to implant according to standard clinical practice. The test grafts were prehydrated by immersing in heparinized saline and cycling vacuum until bubbles were no longer visible. The prehydration step ensured that all the air in the pores was displaced.
[0083]The grafts were implanted bilaterally in a straight ipsilateral configuration (distal carotid artery to proximal jugular vein). The animals ...
example 2
Surface-Modified EPTFE Vascular Grafts in AV Shunt Model
Materials:
[0093]Control grafts (N=2) were ePTFE grafts (by Vascutek) with regular wall of 6 mm with no radial reinforcement.
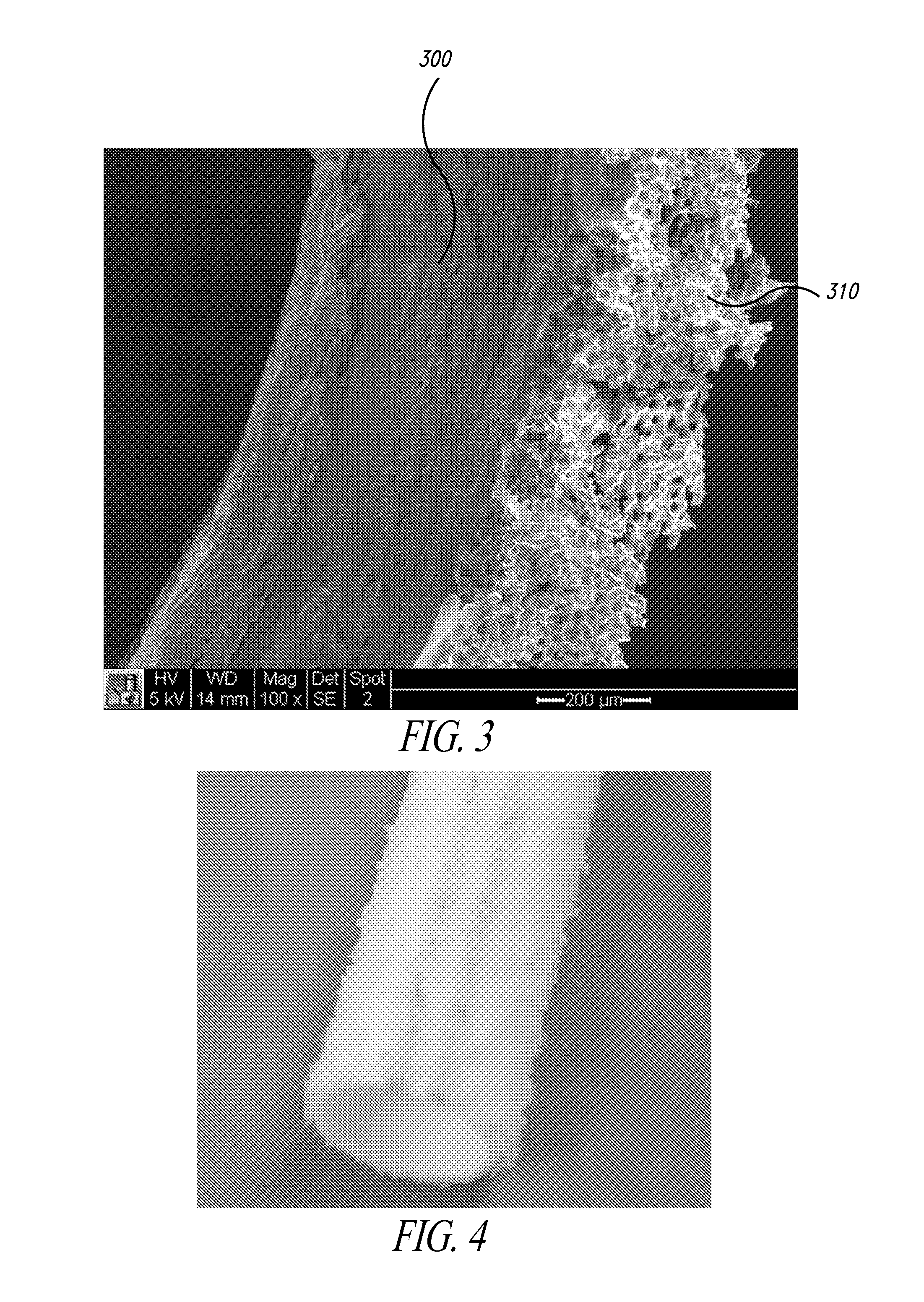
[0094]Test grafts (N=4) were surface-modified ePTFE grafts as shown in FIGS. 3 and 4. In particular, an ePTFE graft was modified with STAR Biomaterial by dip-coating the outer surface with an adhesive (NuSil MED-2214 silicone), and then adhering a monolayer of granules of sphere-templated microporous silicone. These STAR-treated ePTFE grafts had a surface topography formed by ˜300-micron size microporous granules having 35-micron spherical pores, which were interconnected by about 15-micron interpore openings.
[0095]Sheep were 65-80 kg at time of implant. The animals were placed on antiplatelet therapy (salicylic acid and clopidogrel) for the duration of the study.
Methods
[0096]Vascular grafts were implanted in an ovine arteriovenous (AV) shunt model. As in Example 1, the control grafts were soaked in hepari...
example 3
Surface-Modified EPTFE Vascular Grafts in Arterial Bypass Model
Methods:
[0106]Control grafts (N=2) were ePTFE grafts (by Vascutek) with regular wall of 5 mm with no radial reinforcement.
[0107]Test grafts (N=2) were surface-modified ePTFE grafts as shown in FIGS. 3 and 4. In particular, an ePTFE graft was modified with STAR Biomaterial by dip-coating the outer surface with an adhesive (NuSil MED-2214 silicone), and then adhering a monolayer of granules of sphere-templated microporous silicone. These STAR-treated ePTFE grafts had a surface topography formed by ˜300-micron size microporous granules having 35-micron spherical pores, which are interconnected by about 15-micron interpore openings.
[0108]Sheep were 35-40 kg at time of implant. The animals were placed on antiplatelet therapy (salicylic acid and clopidogrel) for the duration of the study.
Methods
[0109]The vascular grafts were implanted in a small caliber arterial bypass model. The control grafts were soaked in heparinized salin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com