Use of tenascin-c as an extracellular marker of tumor-derived microparticles
a tumor-derived microparticle and tenascin-c technology, applied in the field of tumor-derived microparticle isolation and analysis, can solve the problems of wasting time and expense, unable to develop robust methods and tools for early detection and monitoring, and patients are exposed to unpleasant and dangerous side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Tenascin-C as an Extracellular Marker for Tumor-Derived Microparticles
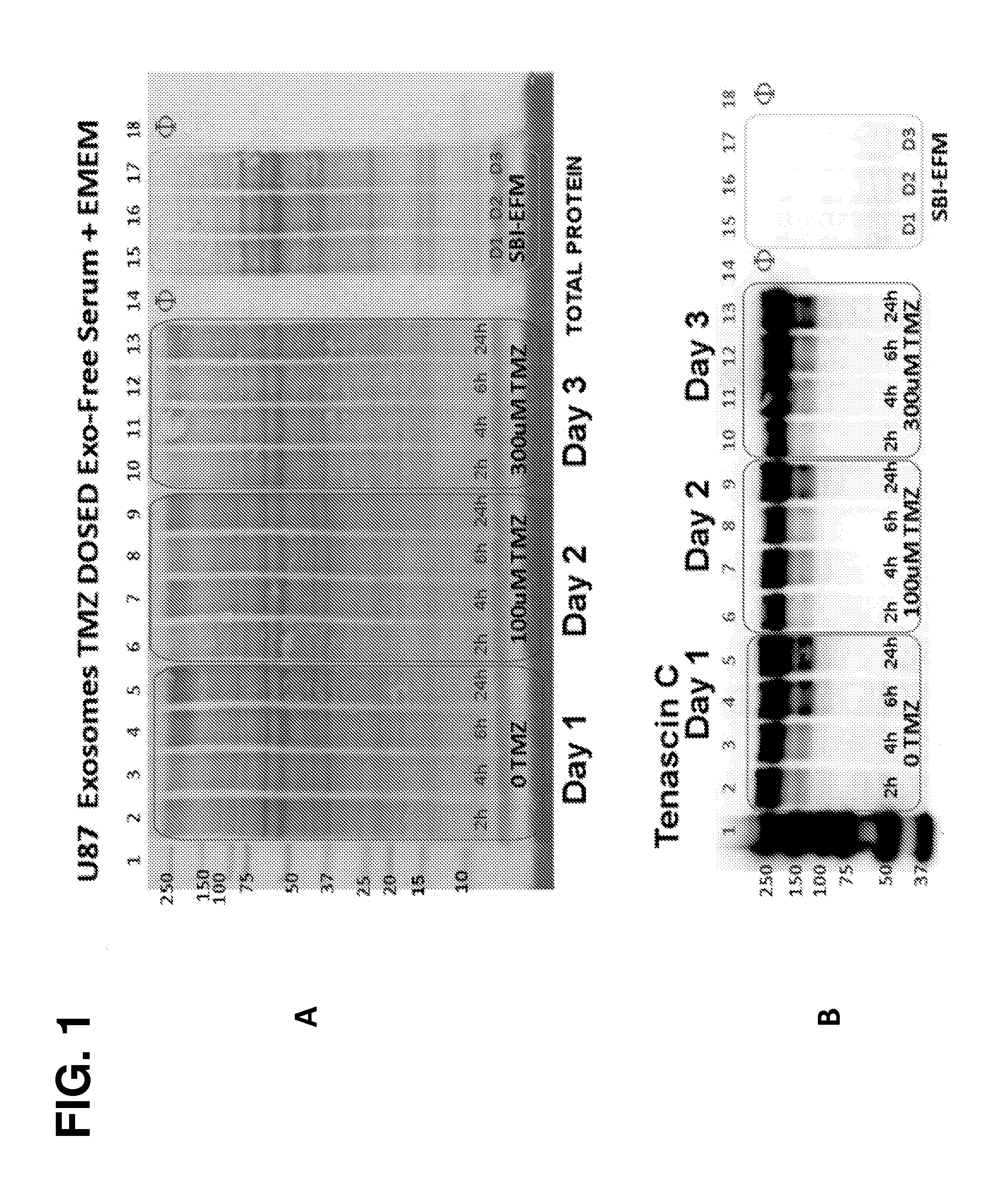
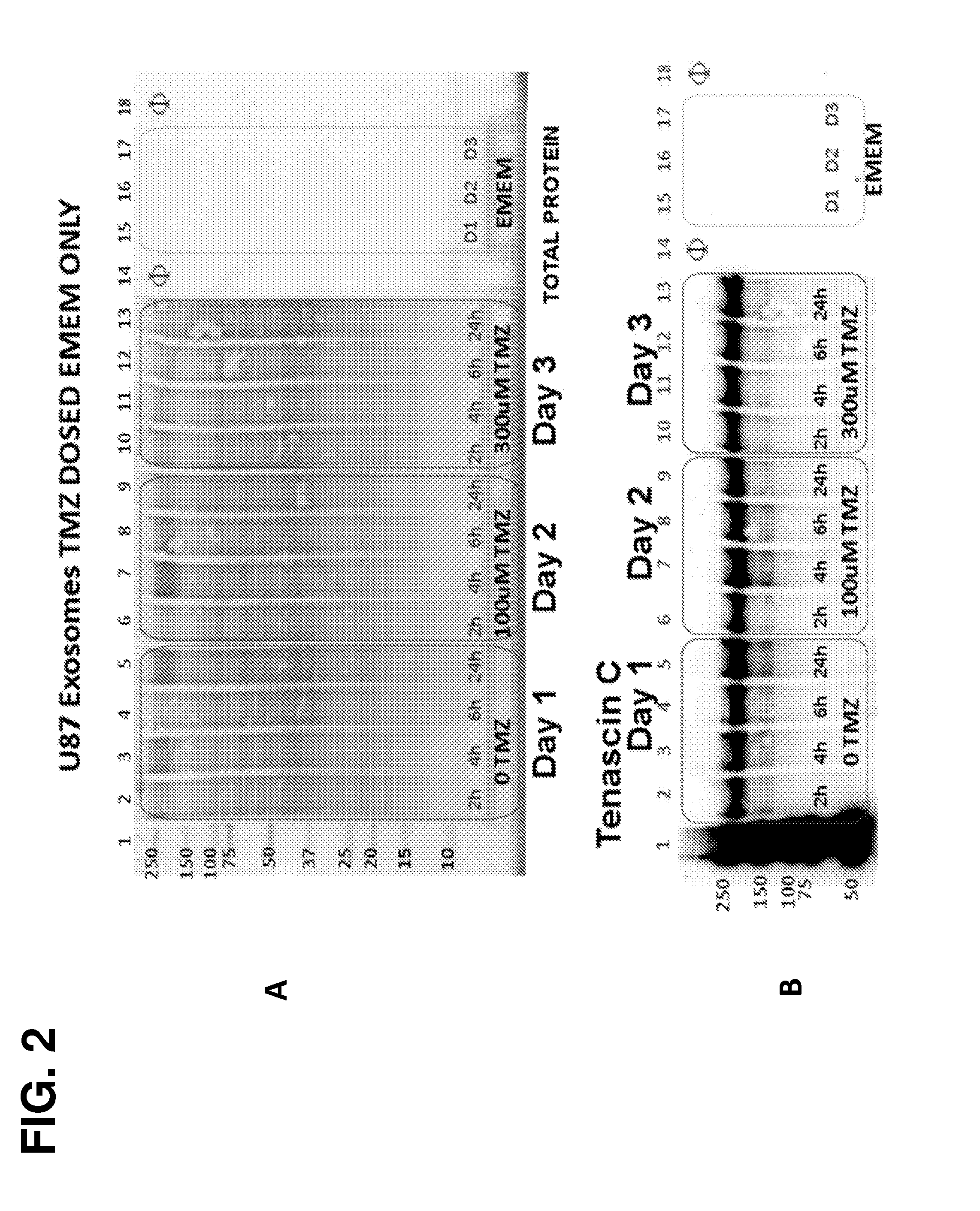
[0178]The following example demonstrates that Tenascin-C is a marker for tumor-derived microparticles. Isolation and analysis of microparticles shed from U87 brain cancer cells under normal conditions, serum-deprived conditions, or treated with the DNA alkylating agent temozolomide (TMZ) revealed robust abundance of Tenascin-C in these tumor-derived microparticles. Robust abundance of Tenascin-C persisted even in the presence of a genotoxic agent (TMZ) and growth of the U87 cells in a nutrient-deprived media.
Materials and Methods
[0179]Overview of Microparticles Harvested from Tumor Cell Lines-Experimental Outline
[0180]U87 MG (ATCC® HTB 14™) cell cultures represent glioblastoma multiforme (GBM). Two Integra CELLine Bioreactors (Hudson, N.H.) were seeded from T-75 cultures of U87 into the 15 ml lower chamber, separated by a 10 kDa MWCO filter from a 500 ml upper reservoir chamber. After growth was established in the...
example 2
Two-Step Recovery of Microparticles Bound to Size Exclusion Chromatography (SEC) Columns and Identification of Tenascin-C
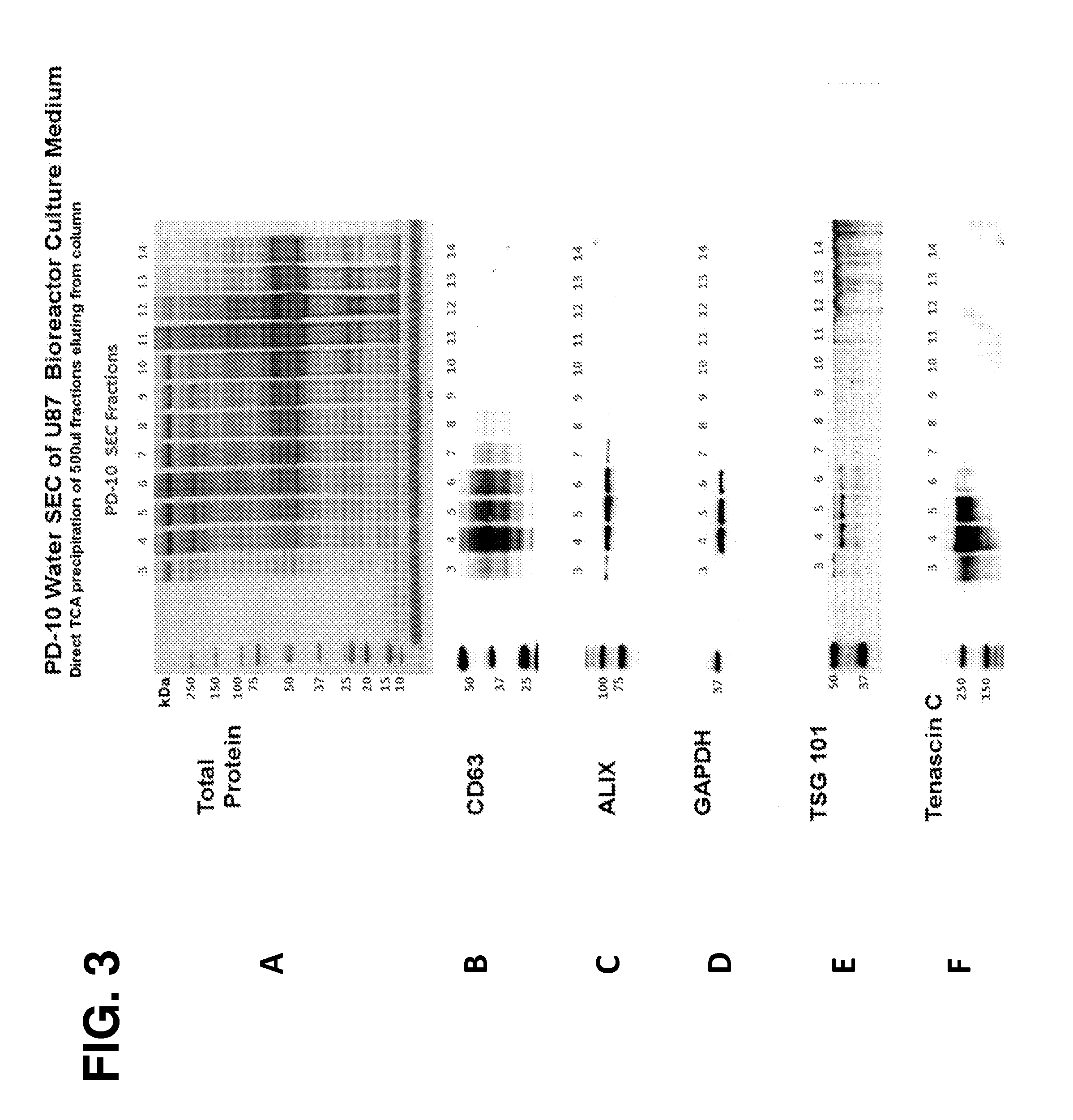
[0218]The following example demonstrates that microparticles may be effectively recovered from a serum-containing sample using a two-step purification method in a size exclusion chromatography (SEC) column. The use of water as a first mobile phase, followed by an arginine-containing solution as a second mobile phase, resulted in controlled elution and separation of microparticles from other sample components. Further, Tenascin-C was identified in the U87-derived microparticle-containing fractions eluted from the column.
Materials and Methods
[0219]PD-10 Fractionation of Serum
[0220]Microparticles to be peptide recovered were prepared by retrieving two separate, 1 ml volumes of glioblastoma U87 bioreactor culture medium from refrigerated storage (containing 0.04% sodium azide and 5 μl / ml of Protease Inhibitor Cocktail III, EMD Millipore). 10 μl of Hdn peptide stock so...
example 3
Direct Affinity Pulldown of Tenascin-C Positive Microparticles from U87 Culture Medium Incubated with Protein Solubilizing Buffer “LaurA”
[0239]The following Example demonstrates the influence of increasing concentrations of a protein solubilizing buffer (“LaurA”) on the protein profile of Tenascin-C and other antigens from affinity captured microparticles from U87 cell culture medium by either the peptide recovered microparticle (PREX) reagent Vn96, or an anti-Tenascin C monoclonal antibody (81C6).
Introduction
[0240]It was seen from Examples 1 and 2 that Tenascin-C protein was robustly associated with U87-derived microparticles, suggesting that Tenascin-C may be used as a robust extracellular marker of tumor-derived microparticles. Without wishing to be bound by theory, it is thought that exposure of Tenascin-C (TNC) on microparticles may be able to be improved by removing background protein contamination. To this end, Applicants explored the use of a protein solubilizing buffer, cal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore diameter | aaaaa | aaaaa |
| pore diameter | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com