Bowel cleansing composition
a technology of bowel cleansing and composition, applied in the direction of drug compositions, antibacterial agents, dispersed delivery, etc., can solve the problems of patient's refusal or avoiding examination, inadequate bowel cleansing, nausea, vomiting, etc., to enhance the ease of administration and compliance, improve the effect of cleansing efficacy and tas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0081]In 100 mL of distilled water as solvent, 45 g of xylitol, 25 g of ascorbic acid, 20 mg of picosulfate, 200 mg of simethicone, 1.4 g of sodium bicarbonate, 3.4 g of potassium bicarbonate, and 20 mg of sucralose were mixed to form 150 mg of a highly concentrated solution, and 350 ml of carbonated water was used as a separate vehicle to prepare a two-part bowel cleansing composition.
[0082]Mixing was conducted by mixing all the ingredients in powder form at the same time and then pouring solvent to dissolve them. Caution is needed because a large amount of carbon dioxide (CO2) gas generated by the neutralization reaction between the acidic ascorbic acid and basic bicarbonate salt causes extensive foaming. Preparing a 1:10 diluted solution of Gasocol containing the anti-foaming agent simethicone beforehand and adding it suitably while mixing can successfully prevent foaming, thereby facilitating the mixing process. Since a pharmaceutical simethicone product containing about 30% of ...
experimental example 1
Safety Tests Concerning the Production of Combustible Gases (In Vitro)
[0085]Measurement of Concentrations of Hydrogen and Methane Gases
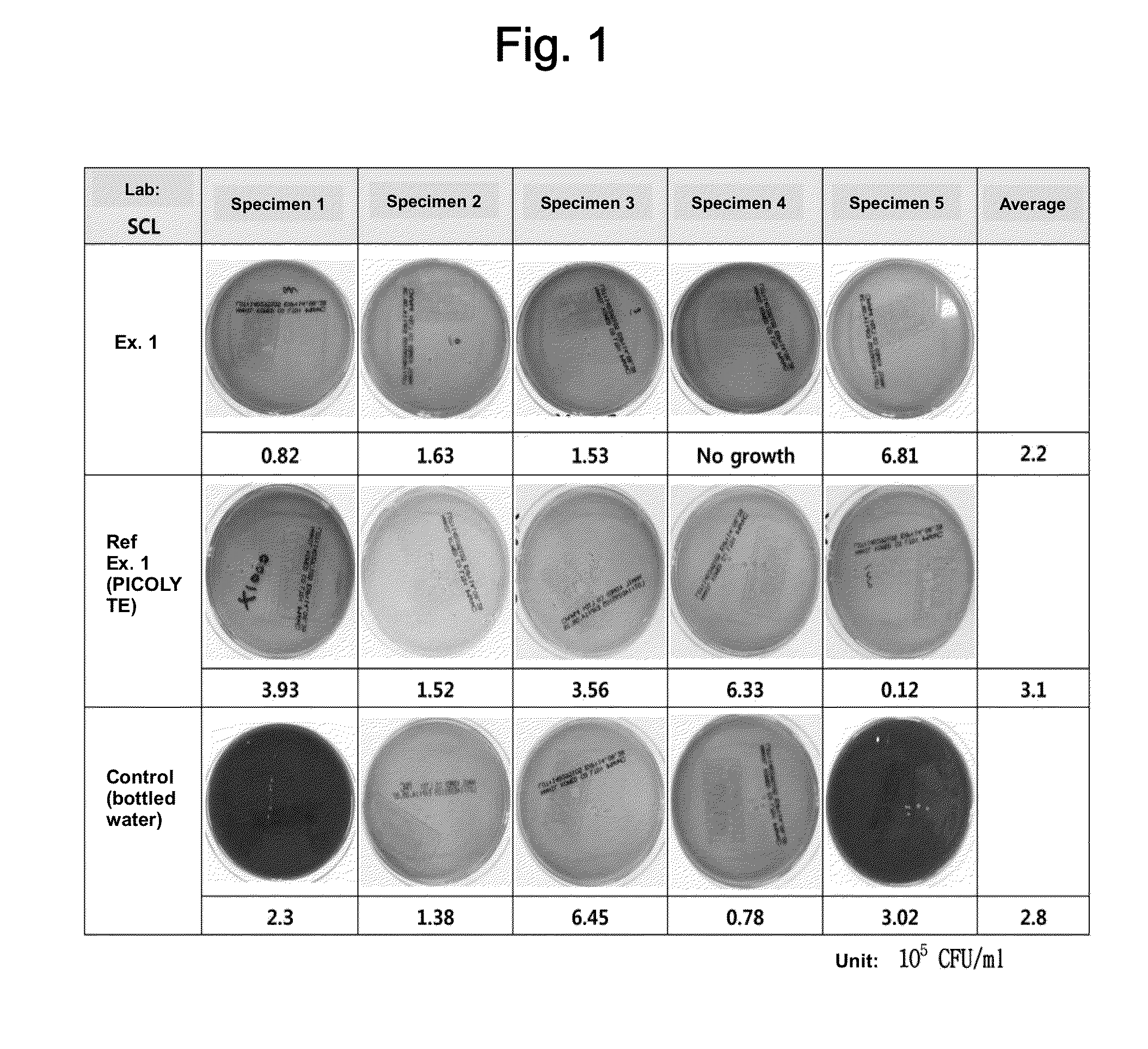
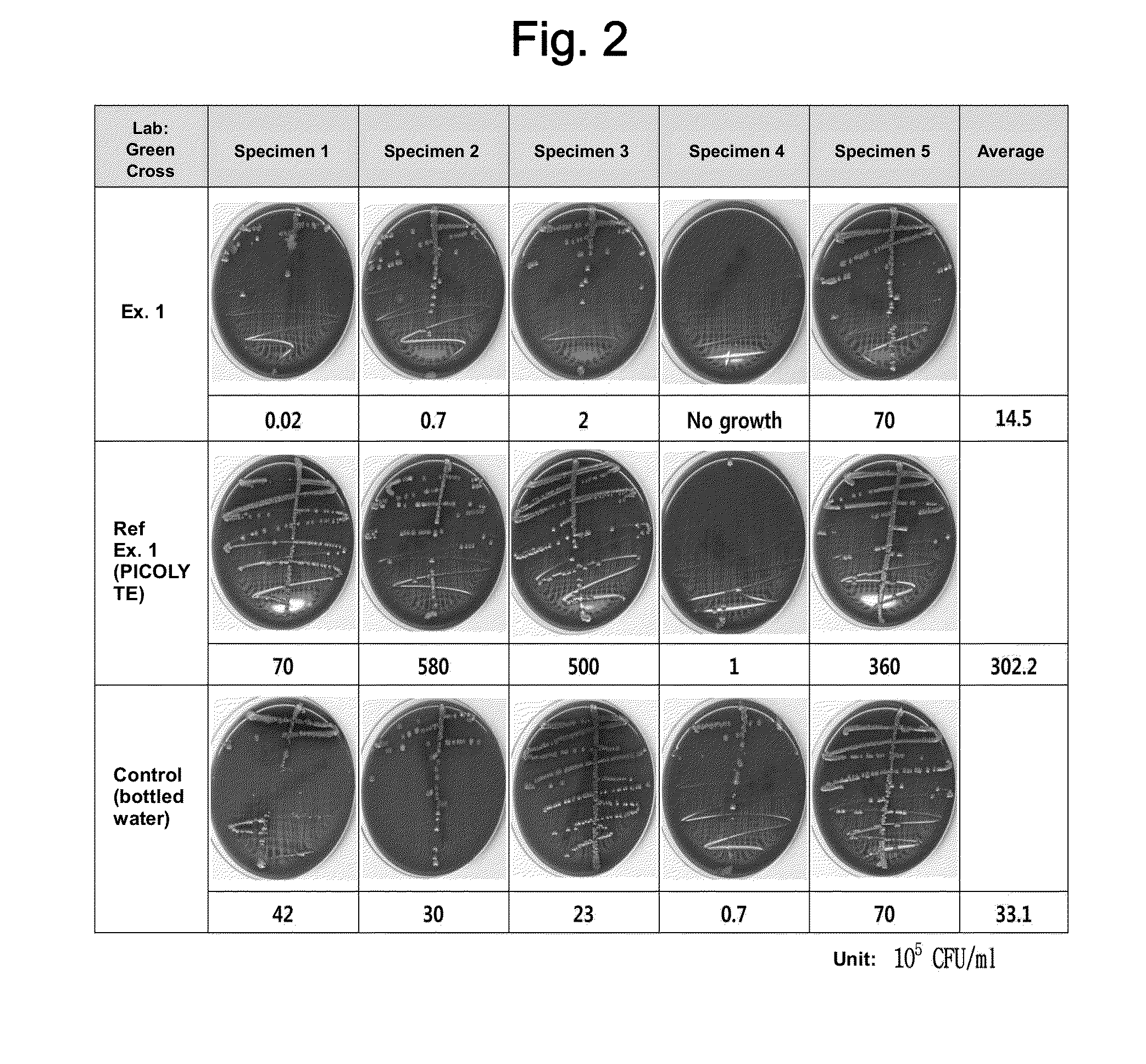
[0086]With regard to safety tests regarding the production of intestinal combustible gases, it is not easy to obtain suitable specimens from the subject or assess colonic gas during colonoscopy and it is not reasonable to conclude that the observed differences are attributable to the difference in the bowel cleansing solution based only on results obtained from a limited number of cases while the conditions of individual subjects after bowel preparation are widely different. Thus, the potential risk of the composition prepared in the Example was indirectly investigated by in vitro tests which can be conducted under identical conditions.
[0087]For this purpose, aliquots of the solutions from Example 1, Comparative Reference Example 1, and Control (bottled water) were mixed with a diluted solution of stool collected from 5 people, and 20 mL of the dilut...
experimental example 2
Bowel Cleansing Efficacy
[0111]Bowel cleansing efficacy was assessed in two aspects: ① colon cleanliness and ② the amount of bubbles.
[0112]Colon Cleanliness
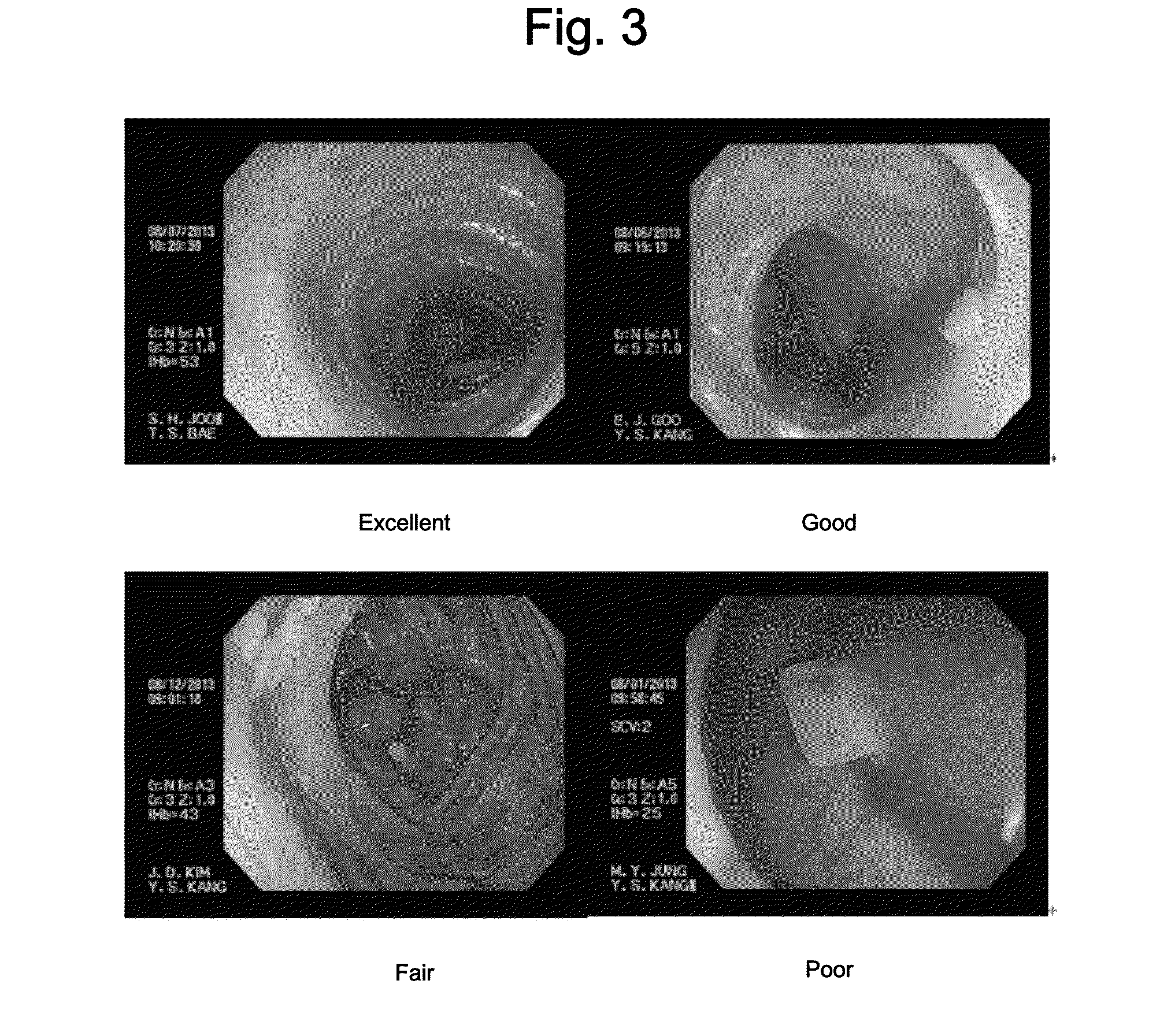
[0113]For the assessment of colon cleanliness, a surgeon evaluated the level of colon cleanliness of a patient using a five-point scale (Excellent, Good, Fair, Poor, Fail) according to the criteria shown in Table 4 and FIG. 3. For a fair evaluation, the evaluation was carried out in a blind test in which the surgeon performing the colonoscopy was not informed of the identity of the bowel cleansing composition consumed by the individual patients.
TABLE 4ExcellentVery thorough cleansing allowing the detectionof even small lesionsGoodSlightly less clean than “Excellent” whilesufficiently clean to allow the detection ofeven small lesionsFairPossible to miss one or two lesions ≦5 mmPoorPossible to miss lesions ≧5 mm due to poorcleanliness but no possibility of missingmalignant lesions, i.e., colon cancerFailRequiring repeat preparation ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com