Compositions and methods for selecting a treatment for b-cell neoplasias
a b-cell neoplasia and treatment technology, applied in the field of b-cell neoplasia treatment selection, can solve the problems of treatment resistance often developing, and achieve the effects of reducing the proliferation of a cell, increasing the expression of ikzf1 or ikzf3, and reducing the expression of ikzf1 or ikzf3
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
DNA Damage Binding Protein 1 (DDB1) and Carbonyl Reductase 1 (CBR1) Bind to Lenolidomide
[0169]Lenalidomide is a highly effective drug for the treatment of multiple myeloma (Rajkumar et al., Blood 106, 4050 (Dec. 15, 2005).) and del(5q) MDS (List et al., N Engl J Med 352, 549 (Feb. 10, 2005)), and its use in a range of other conditions is being actively explored, but the precise mechanism of action of lenalidomide has not been established. In addition, lenalidomide and its analogues thalidomide and pomalidomide have multiple additional biological effects, including stimulation of IL-2 production by T cells, and inhibition of TNF production by monocytes, but the molecular basis of these pleiotropic activities is unknown.
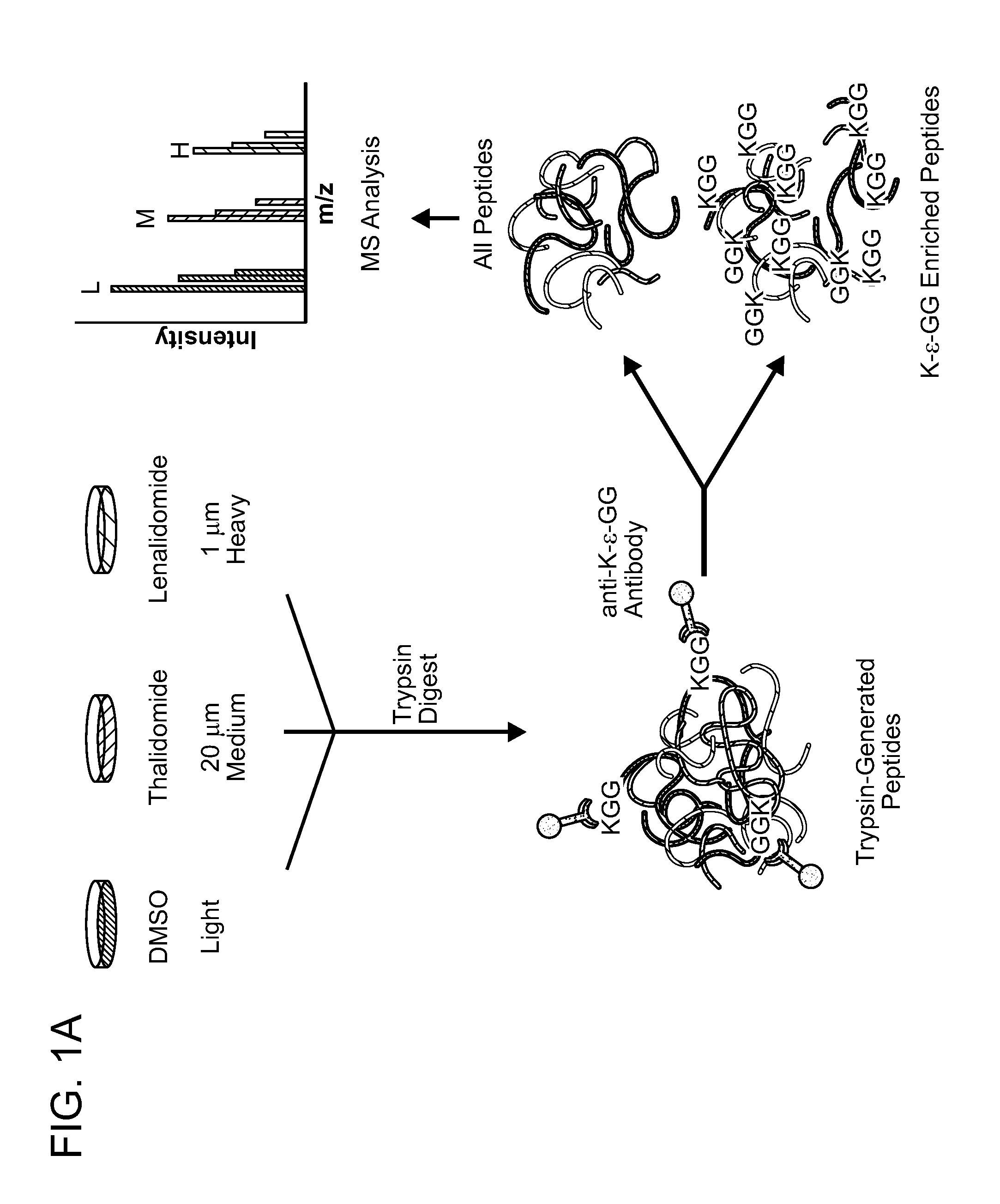
[0170]In order to identify direct protein targets of lenalidomide, a derivative of lenalidomide was synthesized that allowed immobilization of the molecule to a bead (FIG. 2A). This derivative retained the biological activity of lenalidomide, including selective growth...
example 2
Lenalidomide Regulates Ikaros (IKZF1) and Aiolos (IKZF3)
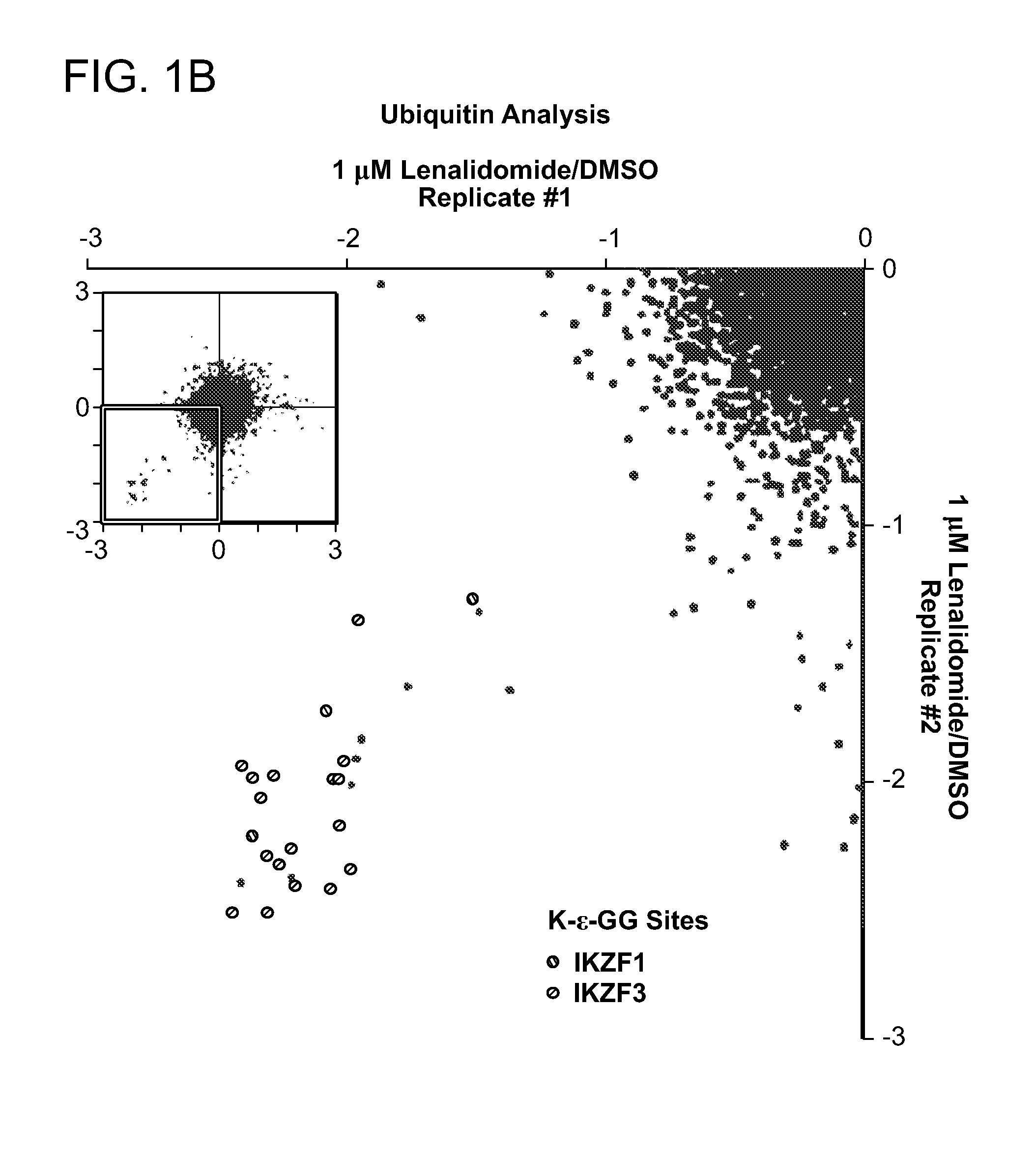
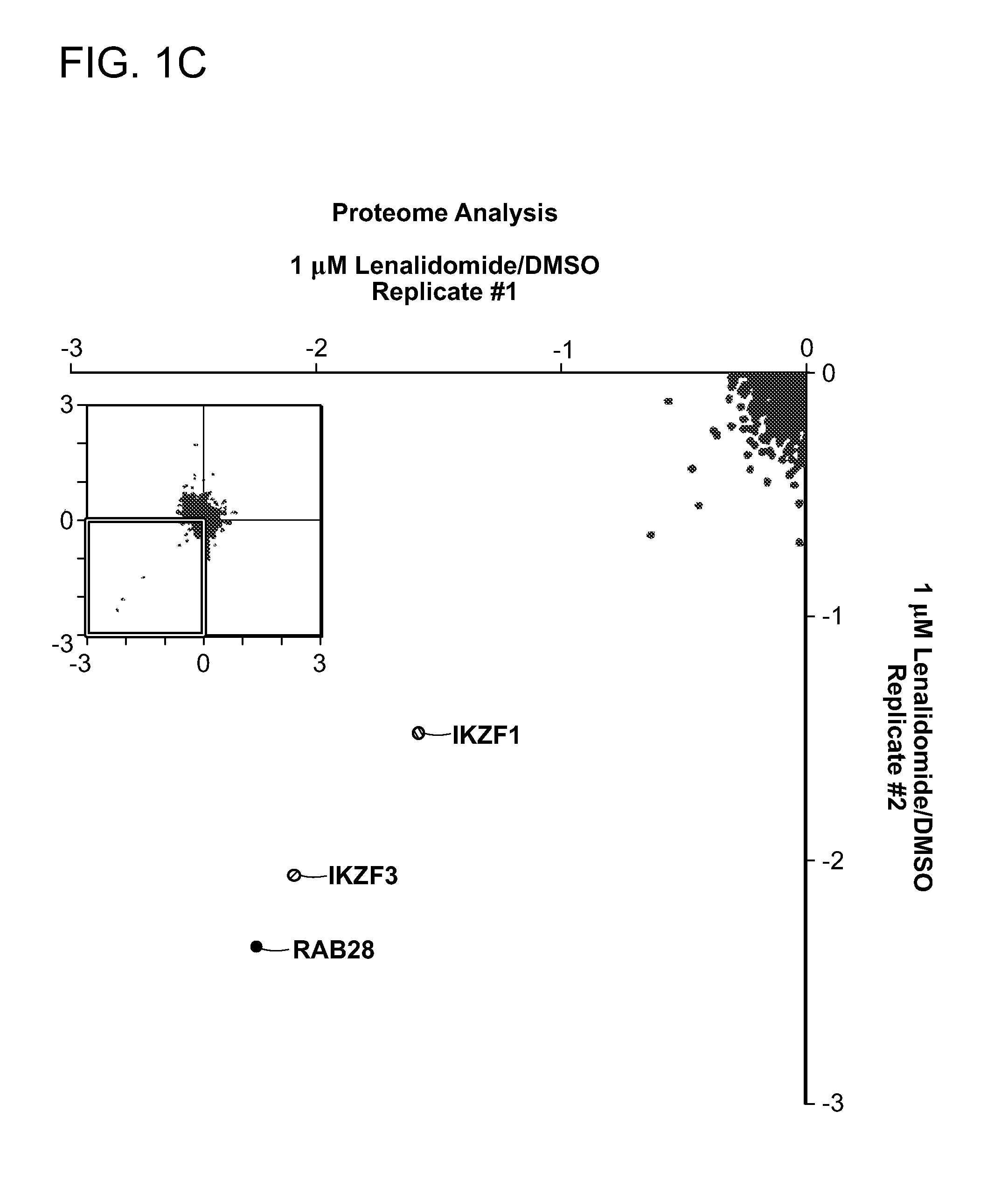
[0173]Two proteins, Ikaros (IKZF1) and Aiolos (IKZF3), scored at the top of the lists of proteins regulated by lenalidomide at both the protein and ubiquitin-site level (FIG. 1C, 1D). Lenalidomide decreased the abundance of IKZF3 (log2 ratio −2.09) and IKZF1 (log2 ratio −1.54). While increased ubiquitination would be expected to be associated with decreased protein abundance, a decrease in ubiquitination of multiple lysine residues of IKZF1 and IKZF3 was observed after treating cells with lenalidomide for 12 hours prior to addition of the proteasome inhibitor MG132. A likely interpretation of these results is that IKZF1 and IKZF3 are rapidly ubiquitinated, targeting them for degradation and thereby resulting in a decrease in abundance of both ubiquitinated and absolute levels of these proteins. IKZF1 and IKZF3 also scored at the top of the list of thalidomide-regulated proteins, consistent with the similar biological activity o...
example 3
Lenalidomide Induced Ubiquitination of IKZF1 and IKZF3
[0177]The direct effect of lenalidomide on ubiquitination of IKZF1 and IKZF3 was assessed. Lenalidomide induced dose-dependent ubiquitination of tagged IKZF1 and IKZF3 in MM1S and 293′ cells (FIG. 8A-8C). Cain-RING ubiquitin ligase (CRL) activity depends on NEDDylation and can be inhibited by the Nedd8 enzyme inhibitor MLN4924. Treatment with 1 μM MLN-4924 prevented the lenalidomide-induced decrease of endogenous IKZF1 and IKZF3 in MM1S cells and of FFluc-fused IKZF3 in 293T cells. These experiments demonstrate that lenalidomide-induced degradation of IKZF1 and IKZF3 involves ubiquitination by a cullin-based E3 ubiquitin ligase.
[0178]Experiments were carried out to determine whether lenalidomide-induced ubiquitination of IKZF1 and IKZF3 is caused by altered binding of these proteins to CRBN, as observed in our proteomic studies. These experiments confirmed that more IKZF1 and IKZF3 co-irnmunoprecipitate with HA-CRBN after 3 hours...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Current | aaaaa | aaaaa |
| Electrical resistance | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com