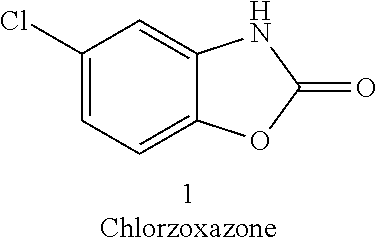
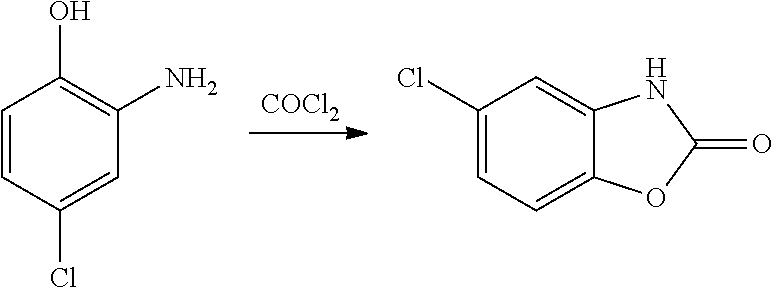
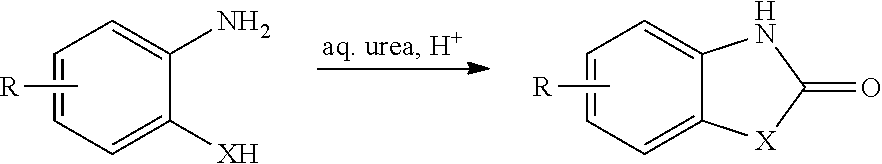Process for the synthesis of chlorzoxazone
a chlorzoxazone and process technology, applied in the field of chlorzoxazone synthesis, can solve the problems of increasing work volume, inefficient industrial scale, and not particularly economical synthesis in terms of energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0036]Ethyl chloroformate (9.8 g, 90.5 mmol) is dripped into a suspension of potassium carbonate (27.9 g, 201.8 mol) and 4-chloro-2-aminophenol (10.0 g, 69.6 mmol) in ethyl acetate (70 mL), heated to 60° C., in 4 hours. After completion of the reaction, the mixture is cooled at 5° C. for 1.5 hours, and the reaction mixture is filtered and re-washed with ethyl acetate (15 mL). The crude product is reduced to a pulp in water for 1.5 hours, filtered under vacuum and washed with water (10 mL); chlorzoxazone (1) (11.1 g), with a purity exceeding 98%, is obtained. Molar yield from 4-chloro-2-aminophenol to chlorzoxazone: 94%.
example 2
[0037]Ethyl chloroformate (2.9 g, 26.3 mmol) is dripped into a solution of 4-chloro-2-aminophenol (3.0 g, 20.9 mmol) and triethylamine (2.7 g, 26.3 mmol) in acetonitrile (20.9 mL), cooled to 0° C. The mixture is left under stirring at 0° C. for one hour, and potassium carbonate (8.4 g, 60.8 mmol) is then added. The resulting mixture is left under stirring at 60° C. for 18 h. After completion of the reaction, the mixture is cooled at 5° C. for 1.5 hours, and the reaction mixture is filtered and re-washed with acetonitrile (5 mL). The crude product is reduced to a pulp in water for 1.5 hours, filtered under vacuum and washed with water (10 mL); chlorzoxazone (1), with a purity exceeding 96%, is obtained. Molar yield from 4-chloro-2-aminophenol to chlorzoxazone: 92%.
example 3
[0038]Ethyl chloroformate (1.96 g, 18.1 mmol) is dripped into a suspension of sodium bicarbonate (5.8 g, 69.0 mmol) and 4-chloro-2-aminophenol (2.0 g, 13.9 mmol) in water (11 ml) at 0° C. The mixture is left to stand at room temperature for about half an hour, after which potassium carbonate is added and the reaction mixture is heated to 55-60° C. The reaction is finished after 2 hours. The reaction mixture is filtered and re-washed with water (10 mL) to obtain chlorzoxazone (1) (2.3 g, 13.6 mol) with a purity exceeding 95%. Molar yield from 4-chloro-2-aminophenol to chlorzoxazone: 98%.
[0039]UPLC-MS [M-H]−=168 m / z
[0040]1H-NMR (in DMSO) (chemical shifts expressed in ppm relative to the TMS signal): 11.82 (1H, s), 7.31 (1H, d), 7.15 (1H, dd), 7.13-7.11 (1H, m). 13C-NMR: 154.7, 142.6, 132.2, 128.2, 121.9, 111.2, 110.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com