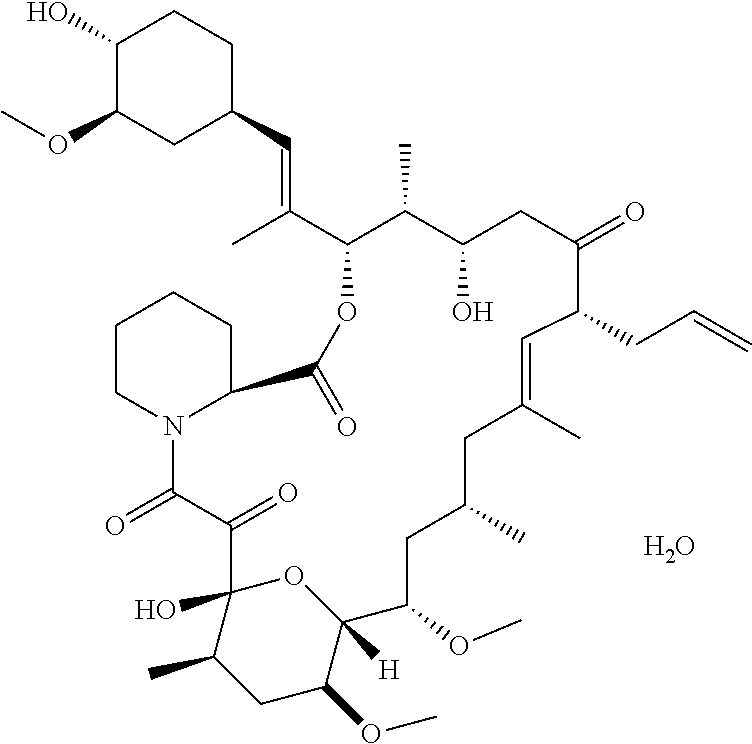
Pharmaceutical composition containing tacrolimus and preparation methods thereof
a tacrolimus and composition technology, applied in the field of pharmaceutical technology, can solve the problems of insufficient absorption rate in the body, reduced drug bioavailability, incomplete dissolution of tacrolimus, etc., and achieve the effect of shortening operation procedures and reducing hazards to operators and the environmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0030]The capsule formulations containing tacrolimus were prepared by reference to the preparation process in example 11 of the patent document CN1820759A. The process is specified as follows:
[0031]Place 1 g of tacrolimus and 5 g of hydroxypropyl methylcellulose (E3) in a beaker, add 25 ml of anhydrous ethanol and 25 ml of dichloromethane, stir to dissolve completely, rotary evaporate the solvents for 25 minutes in a 50° C. water bath until dryness, freeze and dry the mixture for 24 h, take the solids out of the drier, grind, pass the solids through a 80-mesh sieve and obtain tacrolimus solid dispersion.
[0032]Encapsulate the tacrolimus solid dispersion with the strength of 1 mg of tacrolimus.
example 1
[0033]A formula of tacrolimus capsules is shown in the following table:
Percentage ofNamePer capsule (mg)component (%)Tacrolimus11.7Lactose1.62.7Hydroxypropyl methylcellulose11.7Cross-linked sodium0.40.7carboxymethylcelluloseLactose (Additional)5591.7Magnesium stearate11.7(Additional)Ethanol2.5N / ATotal60100.0
[0034]Preparation procedures are as follows:
[0035](1) preparing solution: weighing 24 g of tacrolimus and dissolving the tacrolimus in 60 g of ethanol;
[0036](2) preparing premixed excipients: mixing 24 g of hydroxypropyl methylcellulose, 4.8 g of cross-linked sodium carboxymethylcellulose and 38.4 g of lactose homogeneously using a wet granulating machine;
[0037](3) preparing soft materials: mixing the solution obtained in procedure (1) and premixed excipients obtained in procedure (2) using a wet granulating machine and obtaining soft materials;
[0038](4) drying the soft materials in a vacuum oven at 50° C.;
[0039](5) granulating by forcing the soft materials obtained in procedure ...
example 2
[0040]A formula of tacrolimus capsules is shown in the following table:
Percentage ofNamePer capsule (mg)component (%)Tacrolimus11.7Lactose0.61.0Hydroxypropyl methylcellulose2.54.2Cross-linked sodium0.40.7CarboxymethylcelluloseLactose (additional)54.590.8Magnesium stearate11.7(additional)Ethanol2.5N / ATotal60100.0
[0041]Preparation procedures of the capsules are the same as those of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| mass ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com