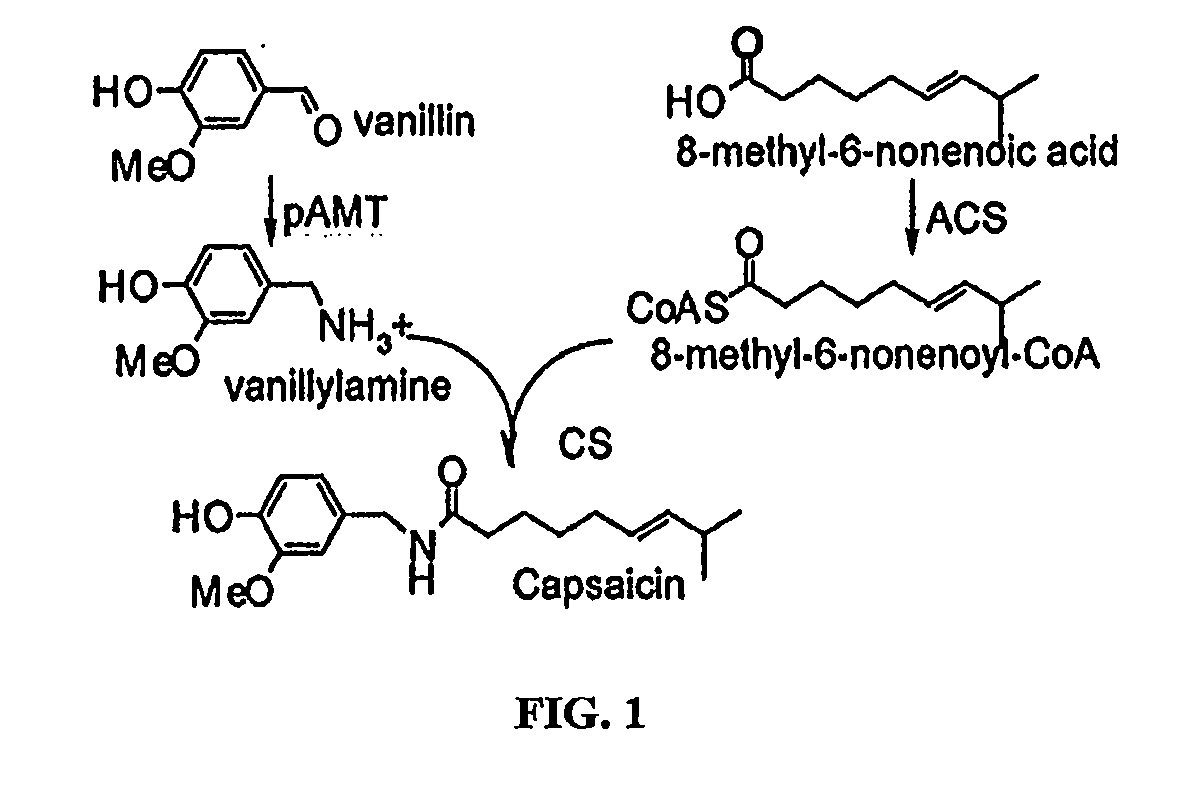
Methods of using acyl-coa synthetase for biosynthetic production of acyl-coas
a technology of acylcoa and synthetase, which is applied in the field of biosynthetic production of acylcoas utilizing acylcoa synthetase, can solve the problems that no biochemical activity has been attributed to any of these proteins, and achieve the effect of facilitating the production of acylcoas and facilitating the subsequent production of capsaicin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Producing Acyl-CoAs
[0032]Applicants amplified ACS1 gene from the cDNA of the green fruits of the ghost chili pepper using the primers of ACS1-sumo-F: CGC GAA CAG ATT GGA GGT GCAACAGATAAATTTATTATTG and ACS1-sumo-R: GTG GCG GCC GCT CTA TTA TCACTTGGTACCCTTGTACAT. The resulting PCR product was purified on 1% agarose gel and mixed with linear pETite N-His SUMO Kan expression vector (Lucigen, Middleton, Wis.). The DNA mixture was used to transform HI-control 10G chemically competent cells by heat shock (Lucigen). The gene insertion was fully sequenced and the encoded amino acid sequence was aligned with that of ACS1 (FIG. 5). As shown in FIG. 5, these two sequences are almost identical except Ile476 in Capsicum annuum ACS1 is replaced by Val in ghost pepper ACS1. The sequence of ghost pepper ACS1 was used to blast Arabidopsis database (http: / / www.arabidopsis.org / ) and identified LCAS4 and LACS5 as its homologues (FIG. 6). As shown in FIG. 6, these three sequences share a sequence i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| flow rate | aaaaa | aaaaa |
| carboxylic acid | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com