Formulations for oral administration of active agents
a technology for oral administration and active agents, applied in the field of drug delivery, can solve problems such as problematic oral administration of peptide pharmaceuticals, and achieve the effect of bioavailability of therapeutically active agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
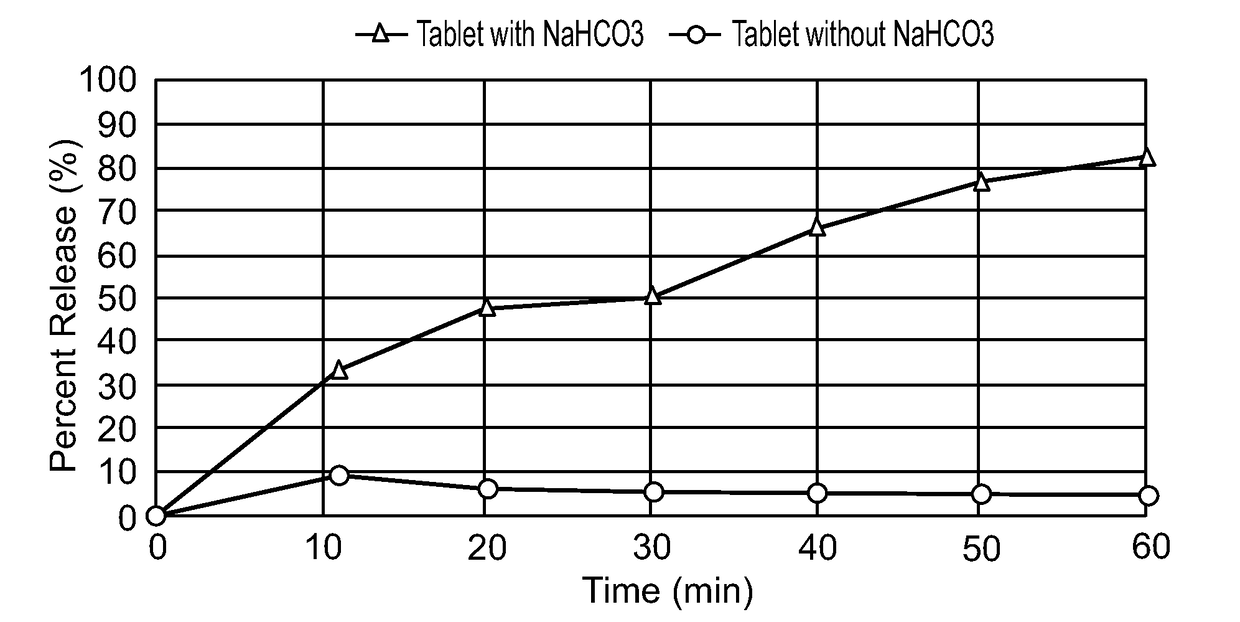
Effect of Antacid on Release of SNAC
[0382]Two tablet formulations were prepared, having the same amounts of SNAC, trypsin inhibitor and teriparatide (parathyroid hormone (1-34)), wherein one formulation further contained 100 mg sodium bicarbonate and the other formulation did not contain sodium bicarbonate. The tablets were in a form of a homogeneous mixture.
[0383]Each tablet formulation was subjected to a dissolution test in 100 ml of simulated gastric buffer (without pepsin), pH 2.0, at 37° C., according to USP 23 Apparatus 2 (paddle) with 50 rotations per minute. The amount of released SNAC in each sample was determined chromatographically, using an HPLC apparatus with Cosmosil™ 5 C18-MS-II (4.6 ID×250 mm) column. Mobile phase consisted of 50% acetonitrile and 50% phosphoric acid solution (0.1%). Flow rate was 1 ml / minute and injection volume was 25 μl. Amount of released SNAC was calculated as a percentage of the amount of SNAC in the formulation.
[0384]As shown in FIG. 8, sodium...
example 2
Effect of Antacid on Pharmacokinetic Profile of Orally Administered Parathyroid Hormone (PTH)
[0386]An open label comparative pharmacokinetic study was performed on ten healthy volunteers. On different visits, each volunteer received the same oral tablet containing 0.75 mg of teriparatide, a recombinant form of parathyroid hormone (1-34) (PTH(1-34)). In the first visit, the tablet was administered with 150 ml water, whereas in the second visit the tablet was administered with 150 ml of 3 mg / ml sodium bicarbonate aqueous solution.
[0387]The formulation was composed of teriparatide (0.75 mg), SNAC (sodium 8-N-(2-hydroxybenzoyl)aminocaprylate), soybean trypsin inhibitor (SBTI) and a small amount of magnesium stearate.
[0388]Tablets were administered in the morning after an 8-hour overnight fast. At each visit a standard meal was provided 3 hours after drug administration. Patients did not eat nor drink alcoholic or caffeinated beverages. There was a two week period between the two visits....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Atomic weight | aaaaa | aaaaa |
| Atomic weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com