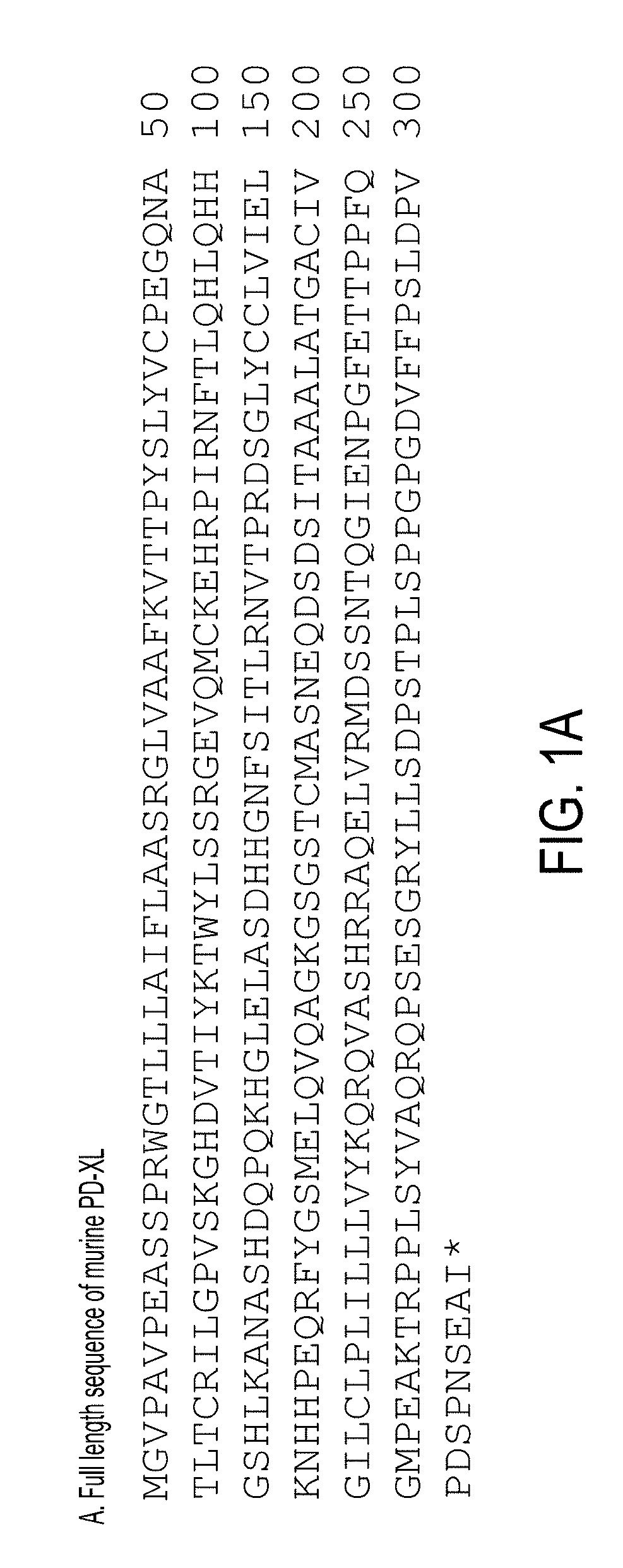
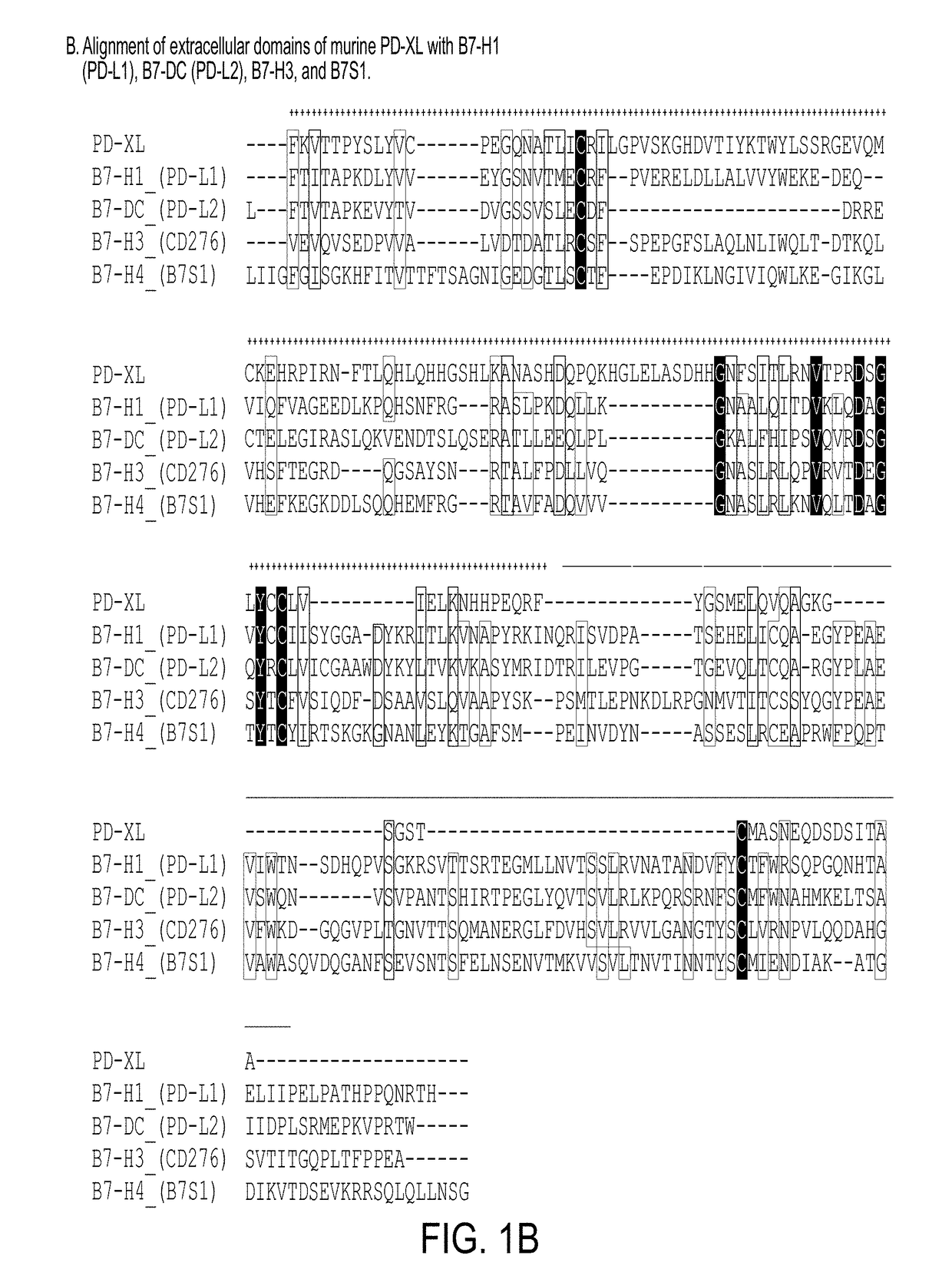
Novel VISTA-Ig constructs and the use of VISTA-Ig for Treatment of Autoimmune, Allergic and Inflammatory Disorders
a technology of vistaig and constructs, which is applied in the direction of peptide/protein ingredients, dna/rna fragmentation, fusion polypeptides, etc., can solve the problems of immune system that cannot distinguish between self and non-self, response that destroys normal body tissues, and cannot adequately control inflammation in ctla-4 ko mi
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cloning and Sequence Analysis of VISTA (PD-L3)
[0668]VISTA (PD-L3) and Treg-sTNF were identified by global transcriptional profiling of resting Treg, Treg activated with αCD3, and Treg activated with αCD3 / αGITR. αGITR was selected for this analysis as triggering of GITR on Treg has been shown to extinguish their contact-dependent suppressive activity (Shimizu, et al. (2002) supra). VISTA (PD-L3) and Treg-sTNF were identified on AFFIMETRIX® DNA arrays based on their unique expression patterns (Table 2). VISTA (PD-L3) exhibited an increase in expression in αCD3 activated Treg and reduced expression in the presence of αGITR; and Treg-sTNF exhibited a αCD3 / αGITR-dependent increase in expression.
[0669]Purified CD4+CD25+ T cells were stimulated in culture overnight with none, αCD3, or αCD3 / αGITR, and RNA isolated for real-time PCR analysis. Expression listed is relative to actin.
TABLE 2Relative ExpressionmRNANoneαCD3αCD3 / αGITRVISTA (PD-L3)6107Treg-sTNF0.20.31.5
[0670]AFFIMETRIX® analysis of...
example 2
Expression Studies of VISTA (PD-L3) by RT-PCR Analysis and Flow Cytometry
[0675]RT-PCR analysis was used to determine the mRNA expression pattern of VISTA (PD-L3) in mouse tissues (FIG. 3A). VISTA (PD-L3) is mostly expressed on hematopoietic tissues (spleen, thymus, bone marrow), or tissues with ample infiltration of leukocytes (i.e. lung). Weak expression was also detected in non-hematopoietic tissues (i.e. heart, kidney, brain, and ovary). Analysis of several hematopoietic cell types reveals expression of VISTA (PD-L3) on peritoneal macrophages, splenic CD11b+ monocytes, CD11c+ DCs, CD4+ T cells and CD8+ T cells, but lower expression level on B cells (FIG. 3B). This expression pattern is also largely consistent with the GNF (Genomics Institute of Novartis Research Foundation) gene array database, as well as NCBI GEO (gene expression omnibus) database (FIG. 3A-3D). See Su, et al. (2002) Proc Natl Acad Sci USA 99: 4465-4470.
[0676]In order to study the protein expression, VISTA (PD-L3...
example 3
Functional Impact of VISTA (PD-L3) Signaling on CD4+ and CD8+ T Cell Responses
[0683]A VISTA (PD-L3)-Ig fusion proteins were was produced to examine the regulatory roles of VISTA (PD-L3) on CD4+ T cell responses. The VISTA (PD-L3)-Ig fusion protein contains the extracellular domain of VISTA (PD-L3) fused to the human IgG1 Fc region. When immobilized on the microplate, VISTA (PD-L3)-Ig but not control Ig suppressed the proliferation of bulk purified CD4+ and CD8+ T cells in response to plate-bound anti-CD3 stimulation, as determined by arrested cell division (FIG. 9A-9B). The VISTA (PD-L3) Ig fusion protein did not affect the absorption of anti-CD3 antibody to the plastic wells, as determined by ELISA, thus excluding the possibility of non-specific inhibitory effects. PD-1 KO CD4+ T cells were also suppressed (FIGS. 9C, 9D), indicating that PD-1 is not the receptor for VISTA (PD-L3). The inhibitory effect of PD-L1-Ig and VISTA (PD-L3)-Ig was also directly compared (FIG. 10). When titr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com