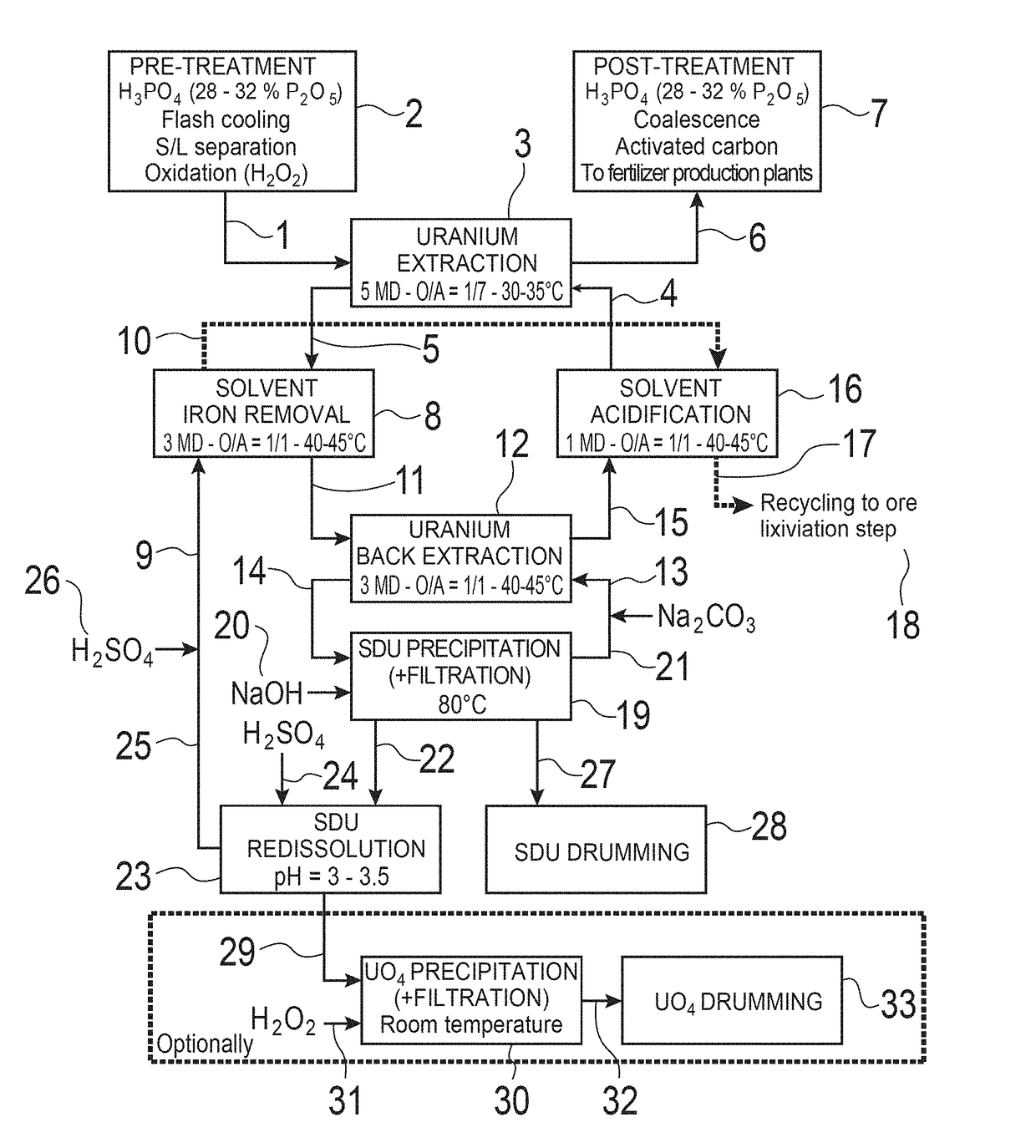
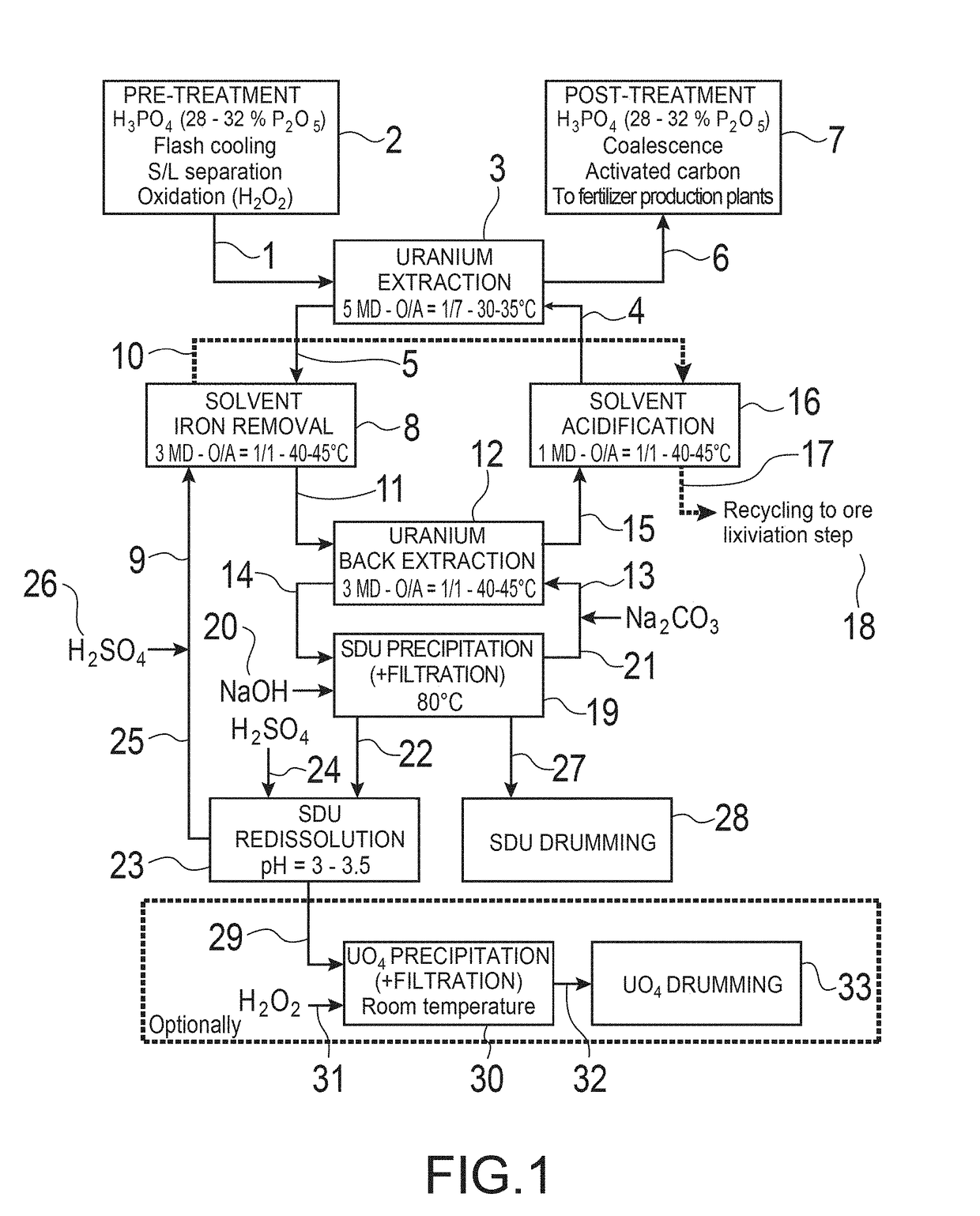
Method for separating iron from an organic phase containing uranium and method for extracting uranium from an aqueous solution of mineral acid containing uranium and iron
a technology of organic phase and uranium, which is applied in the field of method for separating iron, from a liquid organic phase containing uranium and iron, can solve the problems of requiring additional filtration operations, -extraction of impurities such as iron and phosphates, and difficulty in complying with the astm specifications on uranium bearing concentrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0300]In this example, the influence is shown of the initial concentration of uranium in the de-ironing solution used in the method according to the invention for separating iron and from a liquid organic phase.
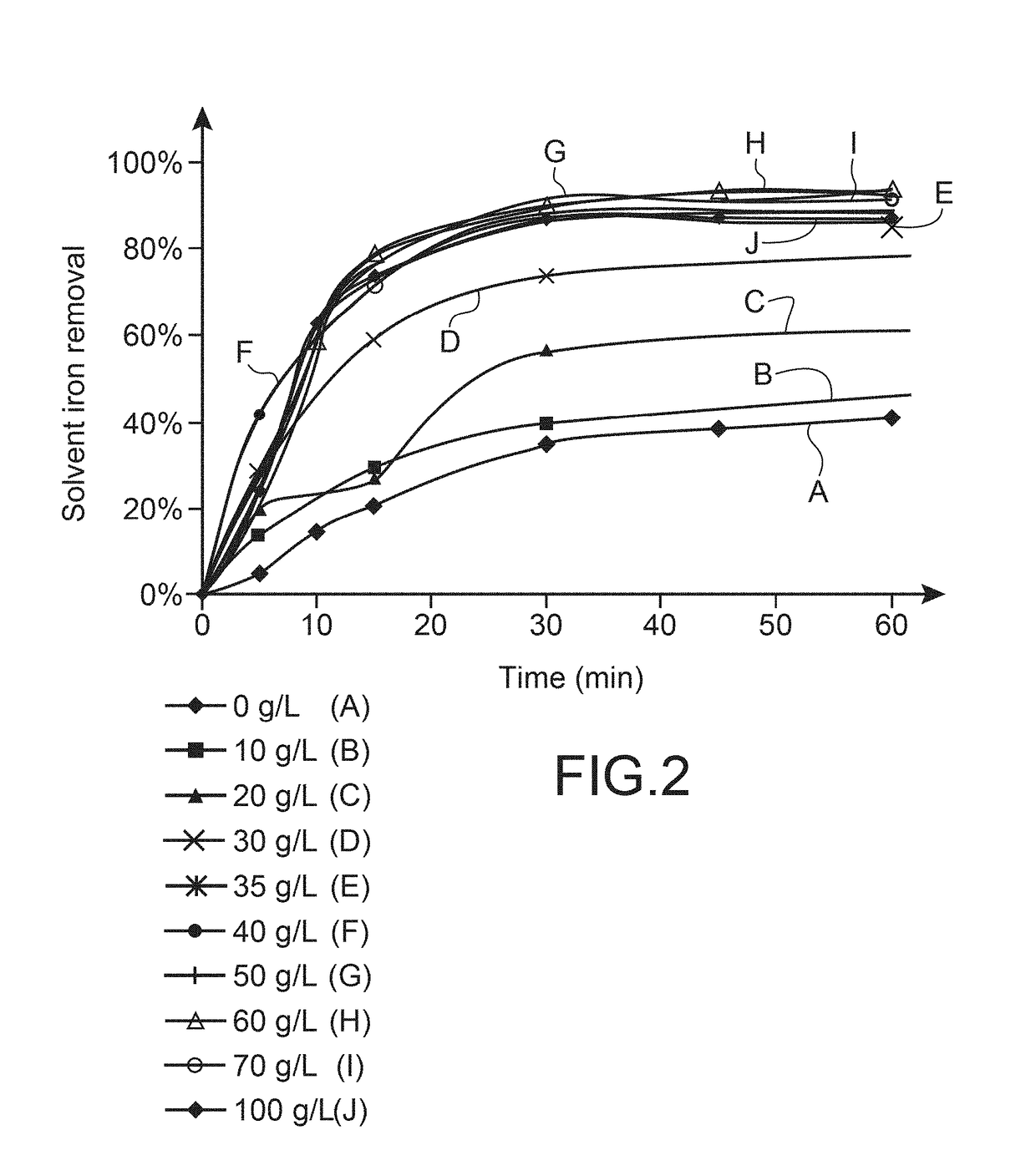
[0301]In order to study the influence of the initial concentration of uranium in the aqueous de-ironing solution, laboratory-scale tests were carried out in separating funnels in the following conditions:[0302]Aqueous iron removal solution (Aqueous phase A): 3 M H2SO4 containing uranium at a variable concentration;[0303]Initial organic phase (Organic phase O), solvent loaded with uranium and with iron: Synergistic mixture of 0.5 M D2EHPA and 0.125 M TOPO in the diluent Isane® IP185, loaded with uranium [U]=1200 mg / L and with iron [Fe]=526 mg / L;[0304]Ratio of O / A phases=1 / 1.[0305]Room temperature (22° C.).[0306]Variable contact time.
[0307]The kinetics of back extraction of uranium and iron from the solvent are determined by analytical monitoring of the concentrations in the aq...
example 2
[0311]In order to complete the preceding data obtained in example 1, complementary, further laboratory-scale tests were carried out in separating funnels in the following conditions:[0312]Aqueous de-ironing solution (Aqueous phase A): 3 M H2SO4 containing uranium at a variable concentration;[0313]Initial organic phase (Organic phase O), solvent loaded with uranium and with iron: Synergistic mixture of 0.5 M Di2EHPA and 0.125 M TOPO in the diluent Isane® IP185, loaded with uranium [U]=1093 mg / L and with iron [Fe]=437 mg / L (The composition of the solvent is thus slightly different from the composition of the solvent in the tests of example 1 where[U]=1.2 g / L and [Fe]=526 mg / L);[0314]Ratio of O / A phases=1 / 1;[0315]Room temperature (22° C.).
[0316]The kinetics of back extraction of uranium and iron from the solvent are determined by analytical monitoring of the concentrations in the aqueous phase after contact with the solvent.
[0317]The results of these complementary, further tests are pr...
example 3
[0324]In this example, tests are carried out in which the method according to the invention for separating iron from a liquid organic phase is implemented in a battery of 3 mixers-decanters (MD) in dynamic counter-current operation.
[0325]The conditions for these tests are the following:[0326]Aqueous de-ironing solution (Aqueous phase A): 3 M H2SO4 ([H+]=5.9 mol / L) containing 39.76 g / L of uranium, with a supply flow rate of 120 mL / h;[0327]Initial organic phase (Organic phase O), solvent loaded with uranium and iron: Synergistic mixture of 0.5 M D2EHPA and 0.125 M TOPO in the diluent Isane® IP185, loaded with uranium [U]=994 mg / L and with iron [Fe]=307 mg / L, with a supply flow rate of 120 mL / h;[0328]Useful volume: 50 ml mixer and 200 ml decanter for stage 3;[0329]Useful volume: 30 ml mixer and 200 ml decanter for stages 1 and 2;[0330]Supply with organic phase in stage 1;[0331]Supply with aqueous phase in stage 3;[0332]Ratio of O / A phases=1 / 1;[0333]Temperature: 40° C. with double-walle...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com