Using fatty acid synthase inhibitors to treat fibrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
FASN Expression in Mouse and Human Lung Tissues
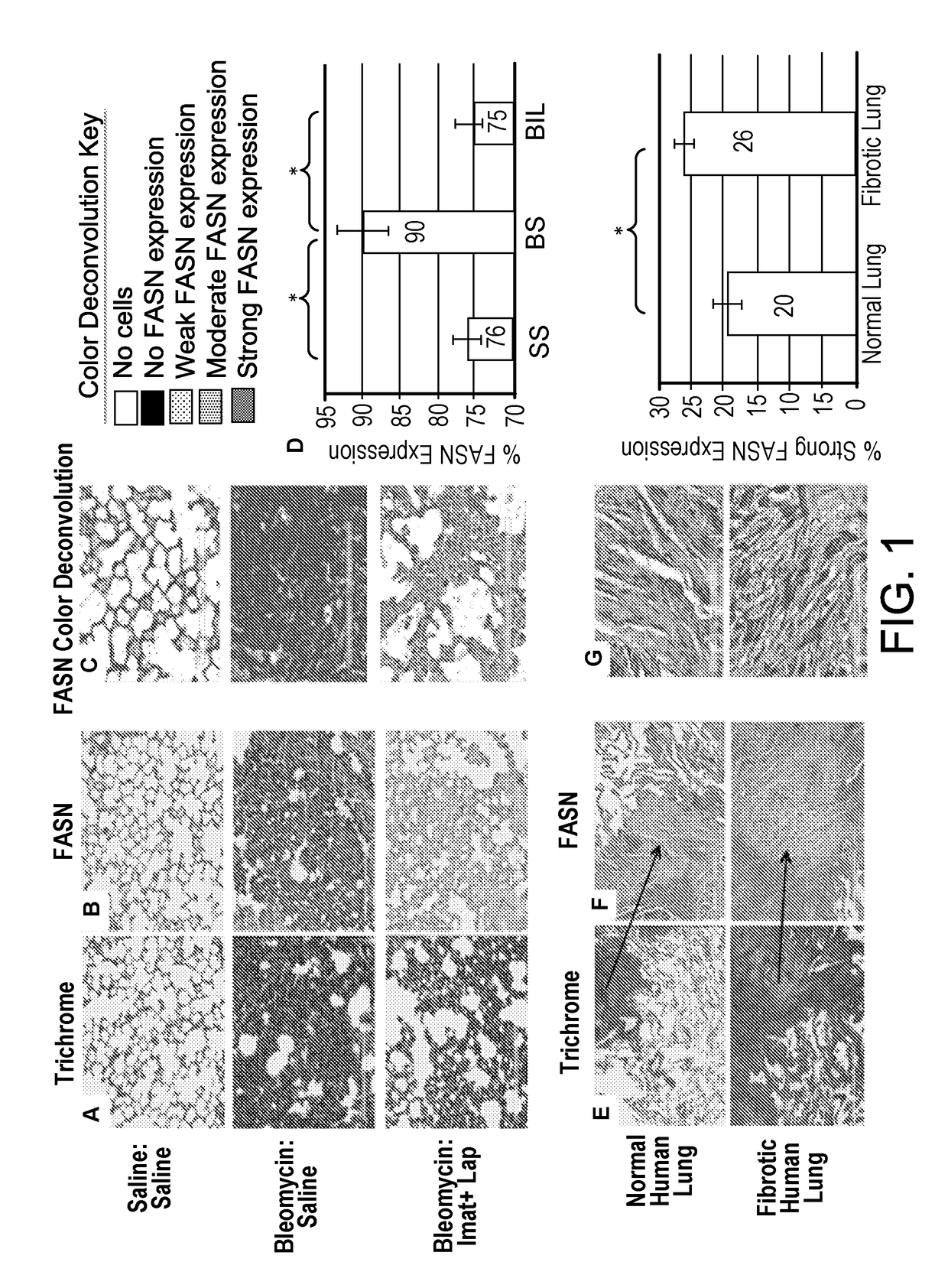
[0047]Mice were treated with bleomycin to induce lung fibrosis or treated with saline as a control. Bleomycin-treated animals were treated dual-treated with lapatinib (an ErbB inhibitor) and imatinib (a PDGF receptor and cAbl inhibitor) to decrease lung fibrosis and stabilize peripheral blood oxygenation. Lungs were harvested and histological sections were prepared. Mouse lungs were stained with Masson's Trichrome (FIG. 1A), or FASN antibodies co-stained with hematoxylin (FIG. 1B). Color deconvoluted images of mouse lungs stained with FASN (FIG. 1C) indicate the area and intensity of FASN staining. Color-coded legend indicating intensity of FASN expression is provided.
[0048]Histological sections of normal and fibrotic lung tissues were stained with Masson's Trichrome (FIG. 1E), or FASN antibodies co-stained with Hematoxylin (FIG. 1F). Arrows indicate the magnified section showing fibroblasts found in normal human lung (Figures E-G top),...
example 2
TGFβ Regulates FASN Expression
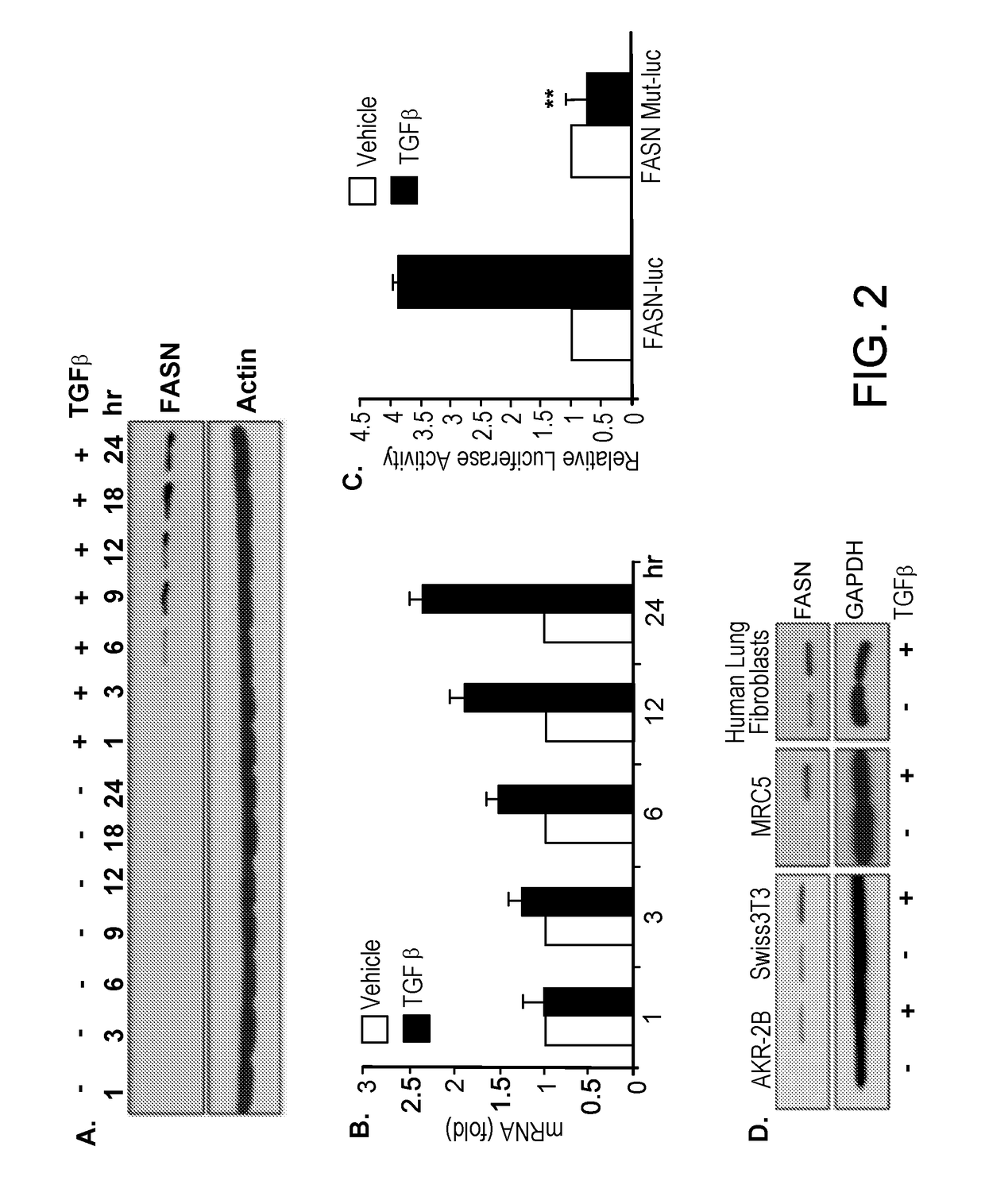
[0051]Quiescent AKR-2B fibroblasts were stimulated in the absence or presence of TGFβ (10 ng / ml). Proteins and total RNA were obtained from treated and control cells. Protein samples were Western blotted for FASN or actin at the indicated times, and RNA samples (500 ng) were analyzed by RT-PCR using FASN primers. TGFβ stimulated FASN expression (FIG. 2A). TGFβ increased FASN transcription (FIG. 2B).
[0052]AKR-2B cultures were transiently transfected with wild-type FASN (FASN-luc) or mutant FASN promoter (FASN-Mut-luc) luciferase constructs and were treated with vehicle or TGFβ (10 ng / ml). Following 24 hours of treatment, normalized luciferase activity was determined. As shown in FIG. 2C, wild-type FASN exhibited significantly more luciferase activity than mutant FASN (n=3; **p-value<0.01).
[0053]Murine (AKR-2B and Swiss 3T3) and human (MRC5) lung fibroblast cells and primary human lung fibroblasts were stimulated for 24 hours in the absence or presence of...
example 3
FASN Induction by TGFβ
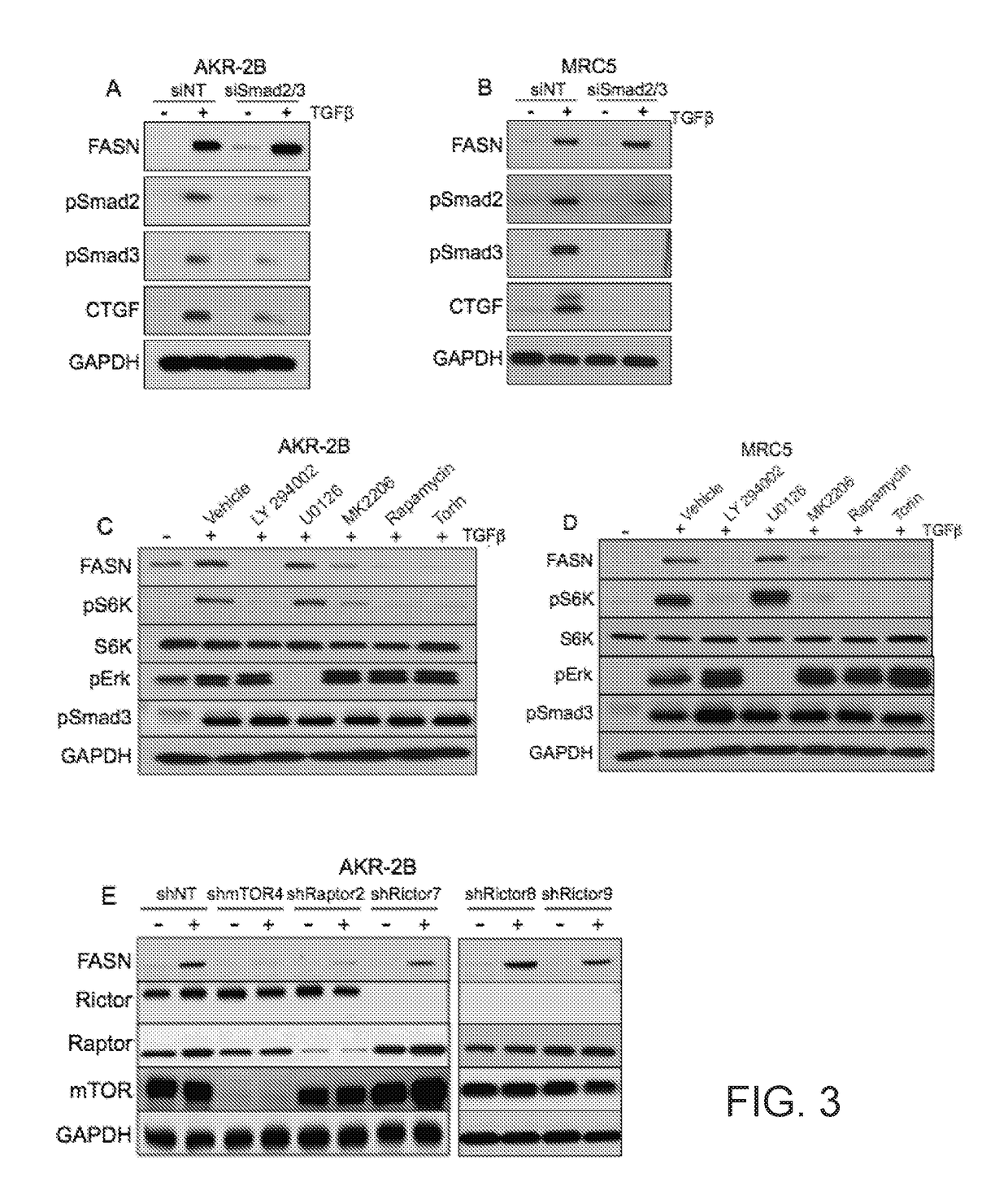
[0055]Murine fibroblasts (AKR-2B) and human lung fibroblasts (MRC5) were transfected with either a nontargeting control siRNA (60 nmol / L) or with Smad2 / 3-targeting siRNA (60 nmol / L). After 72 hours of cultivation, the transient transfectants were stimulated in the absence or presence of 10 ng / ml TGFβ for 24 hours. Proteins were obtained from treated and control cells. Protein samples were Western blotted for FASN, pSmad2, pSmad3, and connective tissue growth factor (CTGF), and Western blotted for GAPDH as a loading control. TGFβ induced FASN expression was independent of pSmad2 / 3 (FIGS. 3A and 3B).
[0056]Murine fibroblasts (AKR-2B) and human lung fibroblasts (MRC5) were stimulated in the absence or presence of TGFβ (10 ng / ml) plus a PI3K inhibitor (LY294002; 10 μM), a MEK inhibitor (U0126; 10 μM), an AKT inhibitor (MK2206; 0.3 μM), a mTORC1 inhibitor (Rapamycin; 100 nM), or a mTORC1,2 inhibitor (Torin; 200 nM). Expression of pErk and pS6K / pS6K was determined at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com