Regulatory T Cell Expressing a Chimeric Antigen Receptor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiments
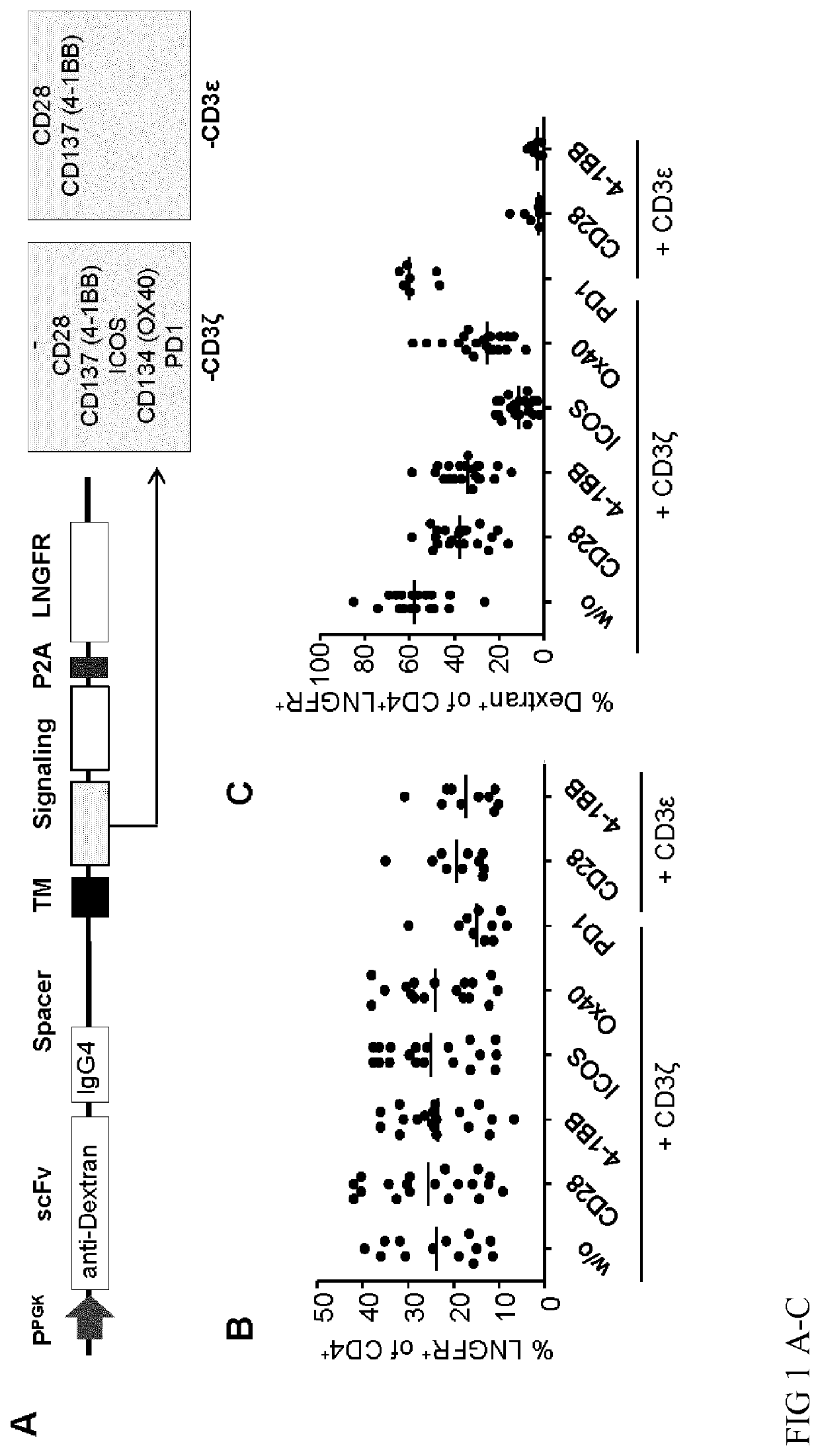
[0200]In one embodiment of the invention a Treg expressing the CAR that comprises the CD3zeta and CD137 signaling domains and an antigen binding domain specific for an exogenous antigen such as dextran is generated. The DNA construct encoding the CAR can be transfected or transduced into a Treg cell by methods well known in the art (e.g. viral-based systems, physical methods, biological methods, chemical methods). Regardless the methods used to integrate, preferentially stably integrate, the nucleic acid encoding the CAR in the Treg cell, as a result the Treg cell expresses the CAR. These Treg cells can be activated in-vitro and in-vivo by addition of dextran to the cells that are in cell culture or circulating in the blood of a subject to whom they have been applied.
[0201]In another embodiment of the invention, Tregs expressing the CAR of the invention are isolated after antigen-specific activation with the respective antigen, e.g. dextran by isolation of cells that express CD137 i...
example 2
Activation of CAR-Tregs With Different Intracellular Signaling Domains
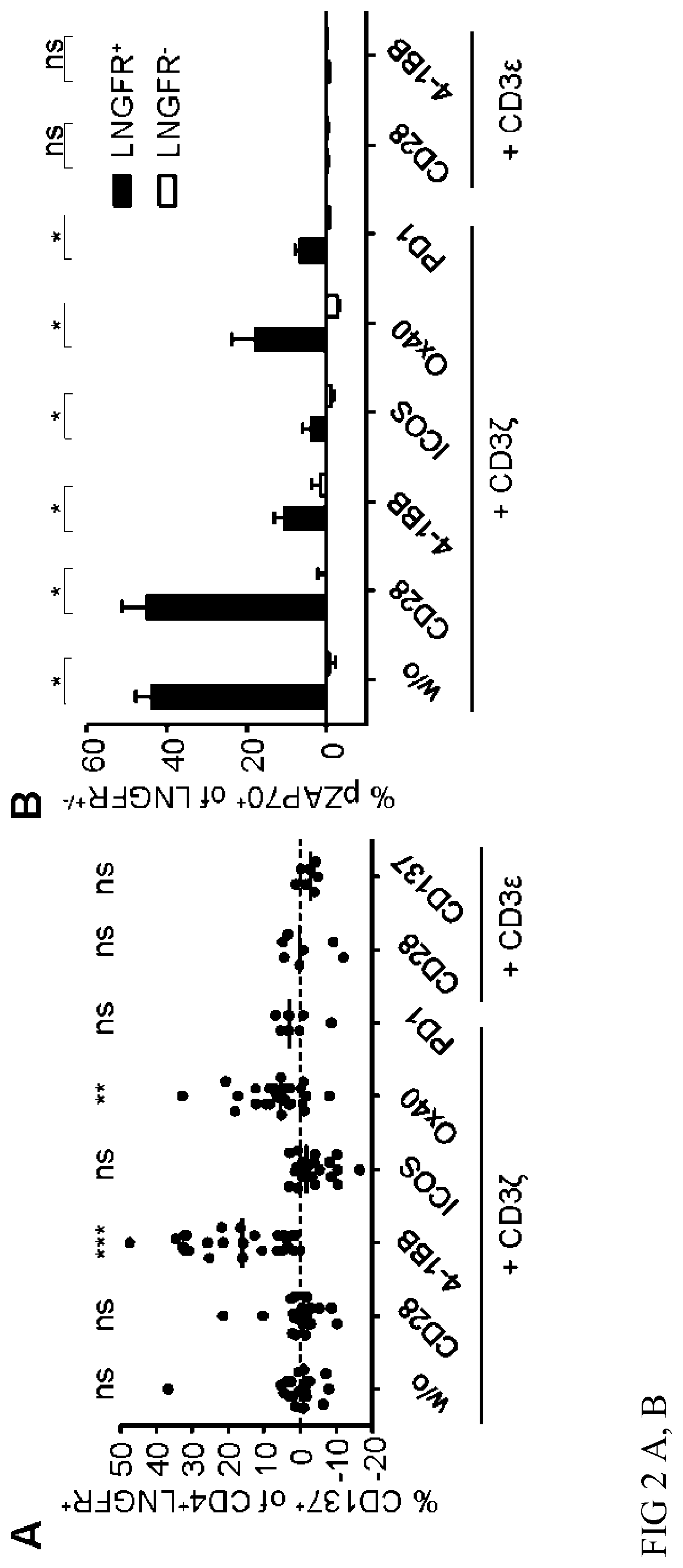
[0212]CAR-Tregs that are specific for an exogenous antigen (e.g. dextran) can be activated by their respective antigen and activation can be analysed by CD137 expression. CAR-Treg activation after stimulation with bead-bound dextran is shown in FIG. 2A. The CD137-CD3ζ-CAR was more potent in inducing CD137 expression in CAR-Tregs (FIG. 2A). Functionality of other tested CAR-constructs with the same specificity was analysed by phosphorylation of ZAP70. Phosphorylated ZAP70 was detected in CAR-Tregs with e.g. CD28-CD3ζ signaling (FIG. 2B), but only the CD137-CD3ζ-CAR induced Treg activation (FIG. 2A).
example 3
Expansion of Dextran-Specific CAR-Tregs With Different Intracellular Signaling Domains
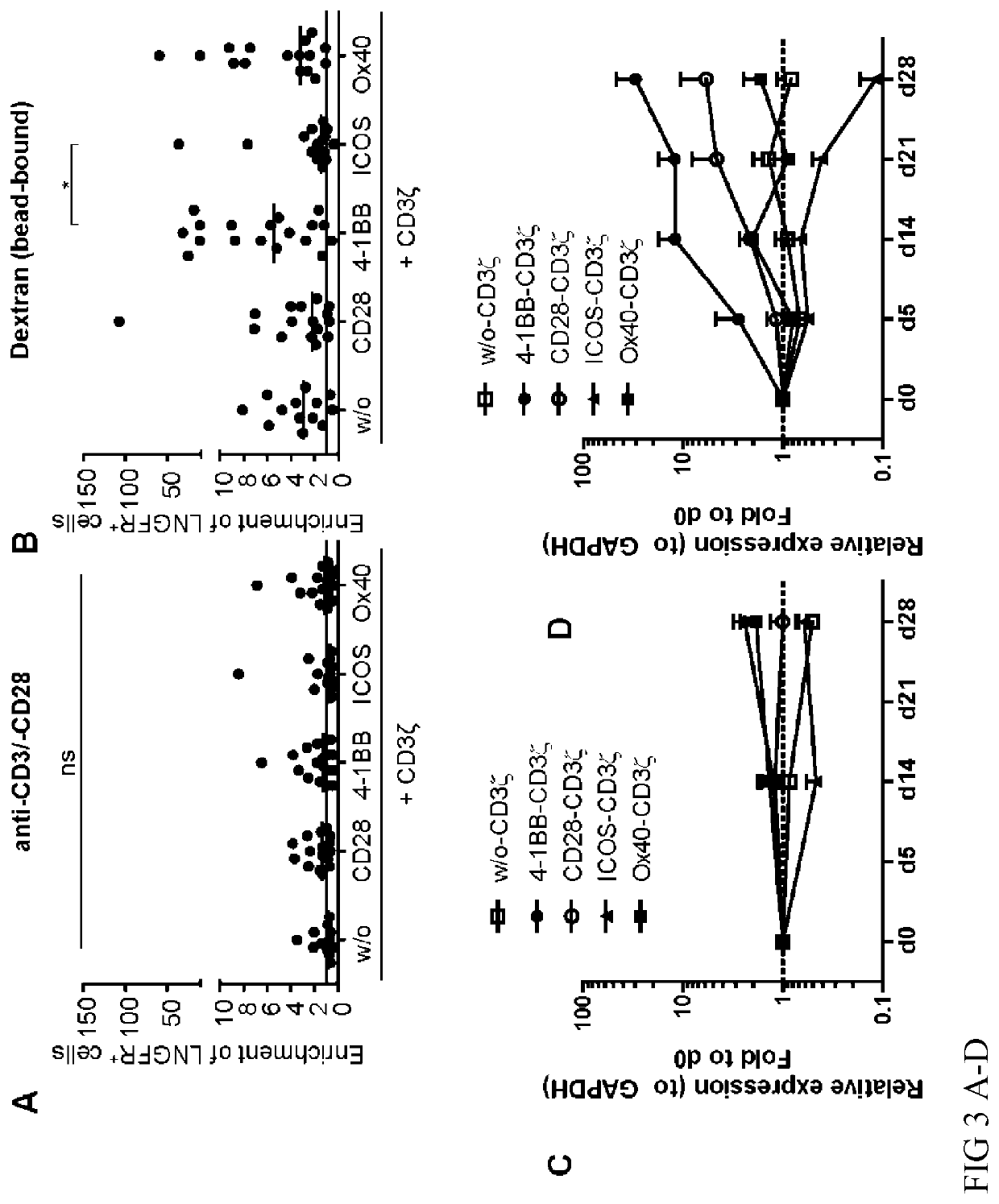
[0213]CAR-Tregs that are specific for an exogenous antigen (e.g. dextran) can be expanded in the presence of anti-CD3 / -CD28 (FIG. 3A, C) or their respective antigen, e.g. bead-bound dextran (FIG. 3B, D). Only CAR-Tregs with the CD137-CD3ζ-CAR expanded showing superior functionality of the CD137-CD3ζ-CAR.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com