Cannabinoid composition and method of sublingual, buccal and oral mucosa delivery
a cannabinoid and oral mucosa technology, applied in the field of cannabinoid composition and method of sublingual, buccal and oral mucosa delivery, can solve the problems of low effective dose for some potential drug recipients, and prior art fails to optimally address the full range of sublingual modalities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
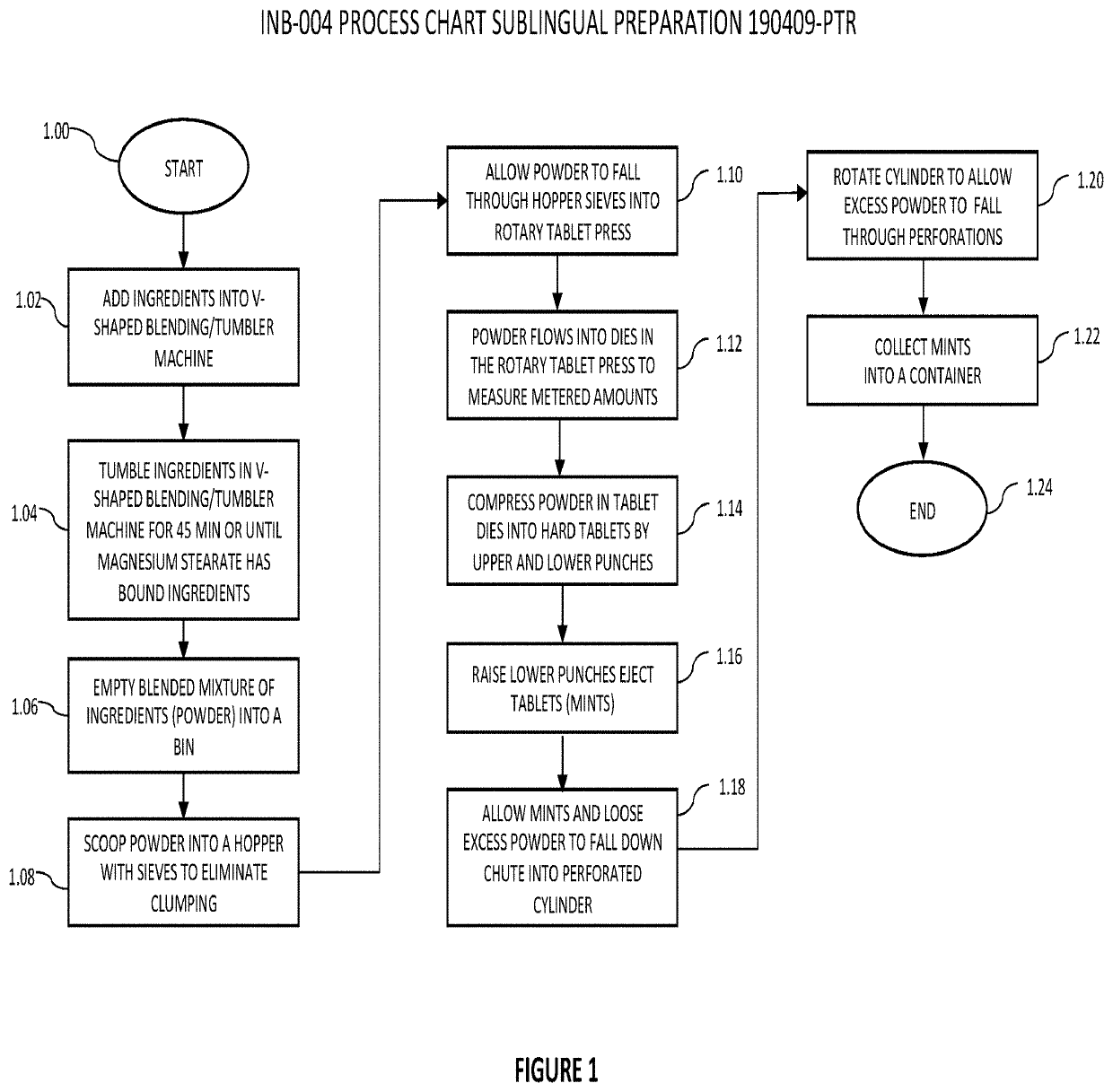
[0025]Referring now generally to the Figures and particularly to FIG. 1, FIG. 1 is a process chart of fabricating various alternate preferred embodiments of the invented formulations in accordance with the invented method.
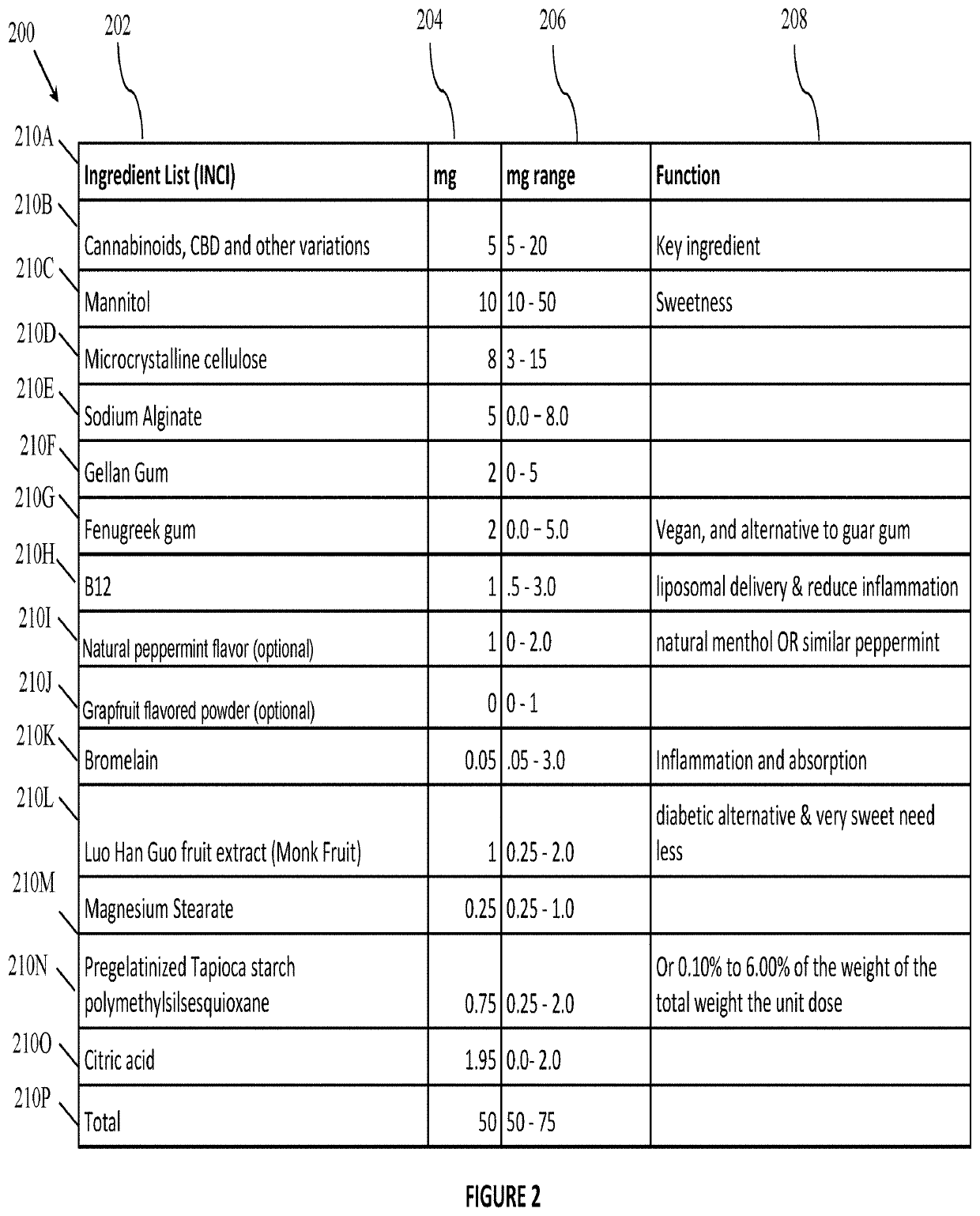
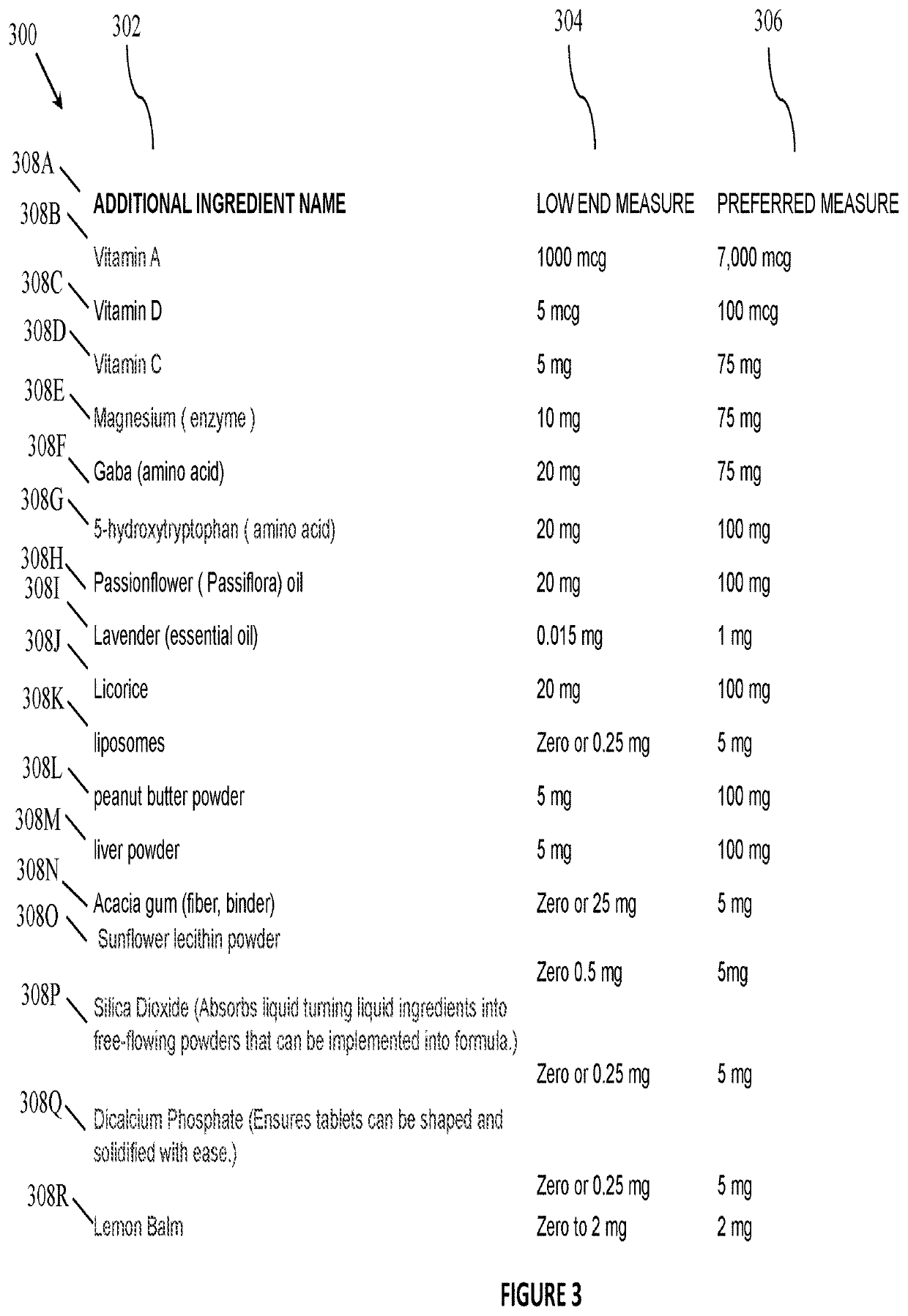
[0026]In a first step 1.00 of the method of FIG. 1, a process of formulating a present mixture is initiated. In step 1.02, the compounder selectively adds one or more of the ingredients disclosed herein into a V-shaped blending or tumbler machine, to include adding two or more substances listed in FIG. 2 or FIG. 3 or otherwise disclosed in the present Application.
[0027]In step 1.04 the compounder utilizes the V-shaped blending or tumbler machine of step 1.02 to tumble the ingredients for a duration of 45 minutes or until the ingredient magnesium stearate has bound the ingredients. In step 1.06 the compounder empties the blended mixture of ingredients (hereinafter the “powder”) out of the blender and into a bin. In step 1.08 the compounder scoops the powder into a h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| total volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| sublingual composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com