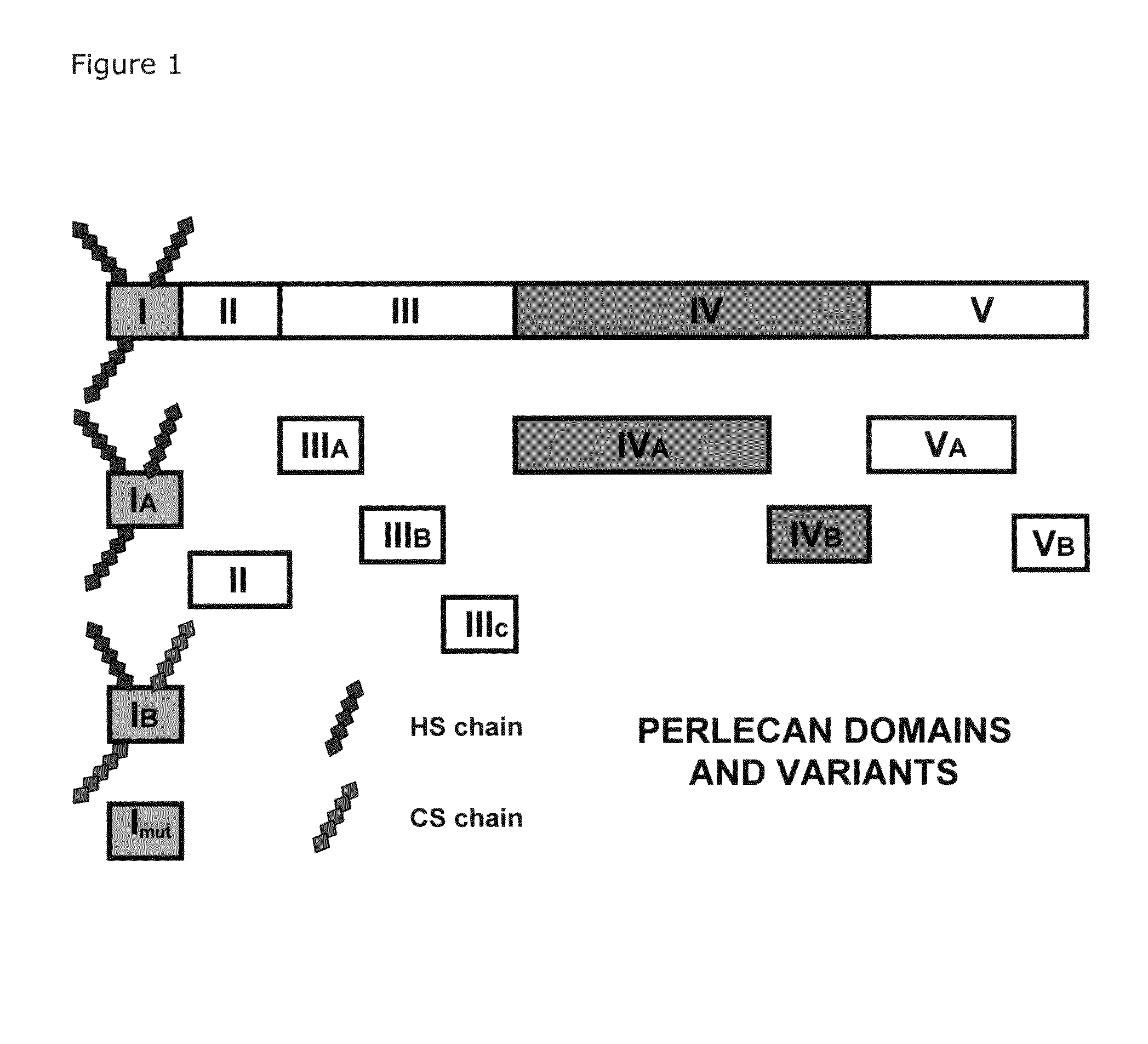
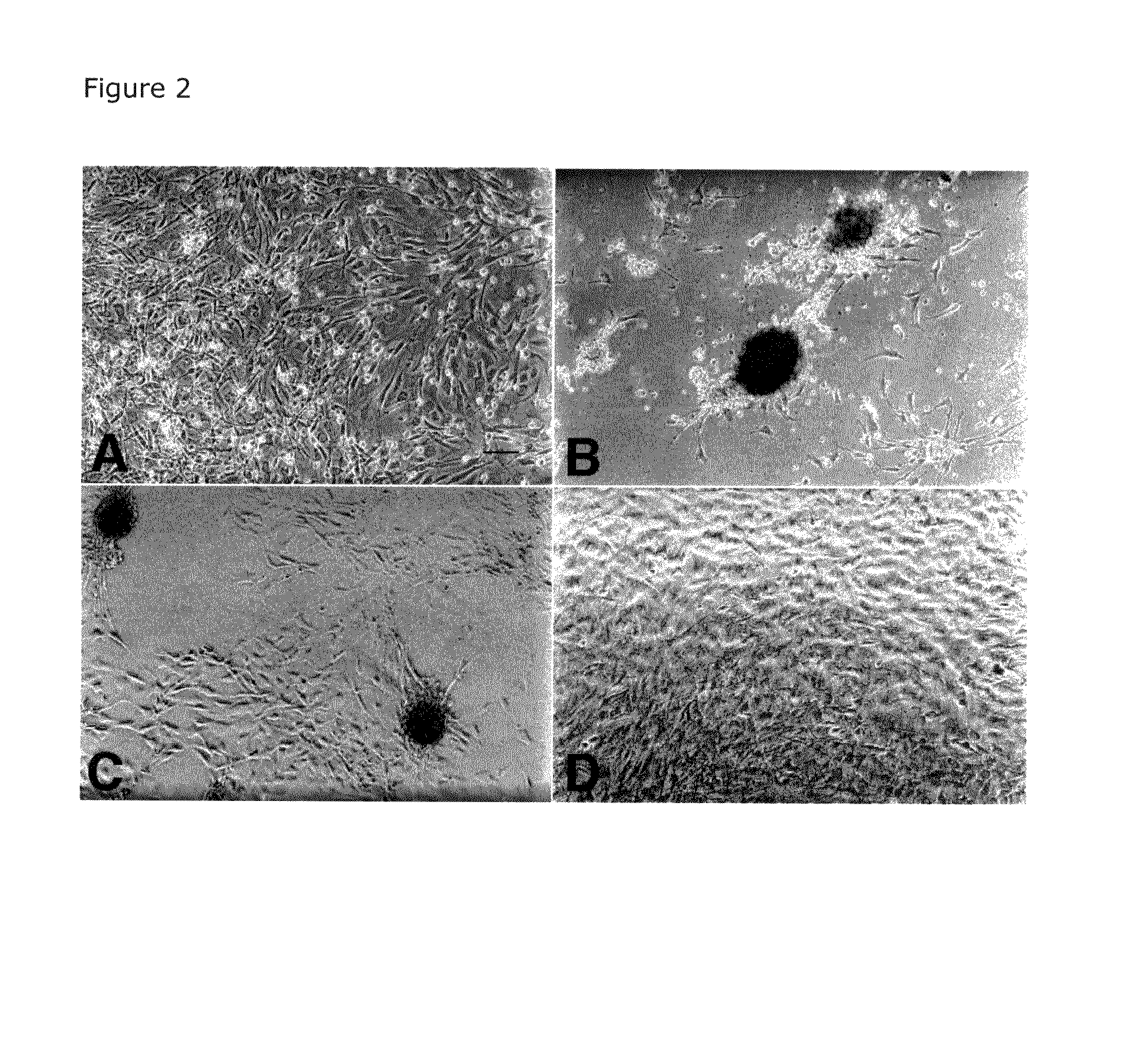
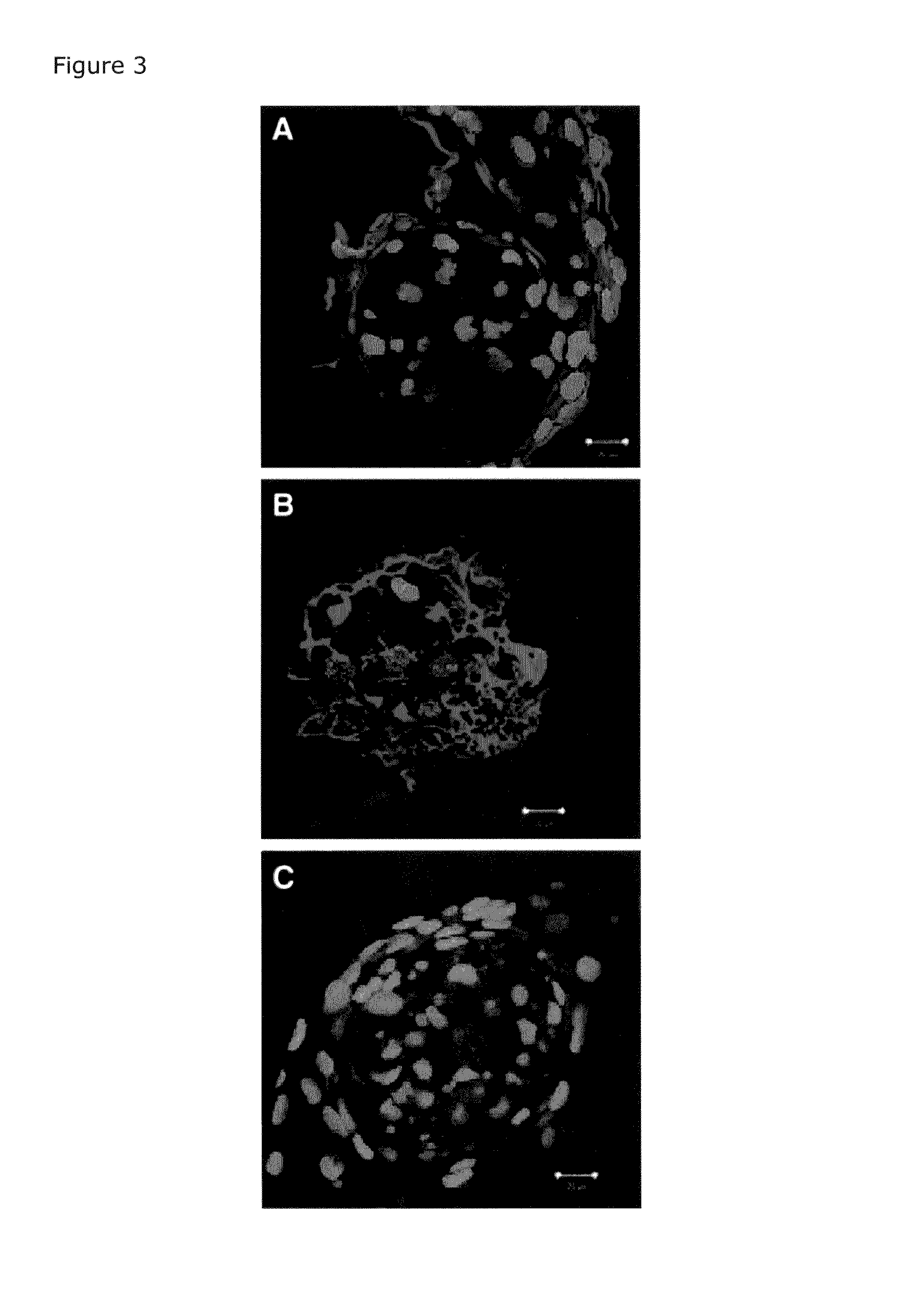
Delivery system for heparin-binding growth factors
a technology of growth factor and delivery system, which is applied in the field of proteoglycans, can solve the problems of defective endochondral ossification, large amount of inventive proteoglycan production, and severe disorganization of the columnar structure of chondrocytes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
Materials
[0042]Pln / HSPG2 was obtained from Becton-Dickinson Labware (Bedford, Mass.). The rabbit polyclonal antibody against rat aggrecan was provided by Dr. Kurt Doege (Shriner's Hospital for Children, Portland Unit). The rabbit anti-mouse antibody against type X collagen (PXNC1-88) was provided by Dr. Greg Lunstrum (Shriner's Childrens Hospital, Portland, Oreg.) The rabbit IgG antibody against mouse type II collagen was purchased from Biodesign International (catalog # T40025R). Species-specific, Texas-Red conjugated secondary antibodies were purchased from Amersham Corporation (Arlington Heights, Ill.).
Immunofluorescent Detection of Extracellular Matrix Components
[0043]After culture upon matrix for 6 or 9 days, cell aggregates and monolayers were rinsed twice with Dulbecco's phosphate buffered saline (D-PBS) without calcium or magnesium. The specimens were subsequently fixed, washed 3 times (5 minutes at room temperature) with D-PBS and incubated with the pri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com