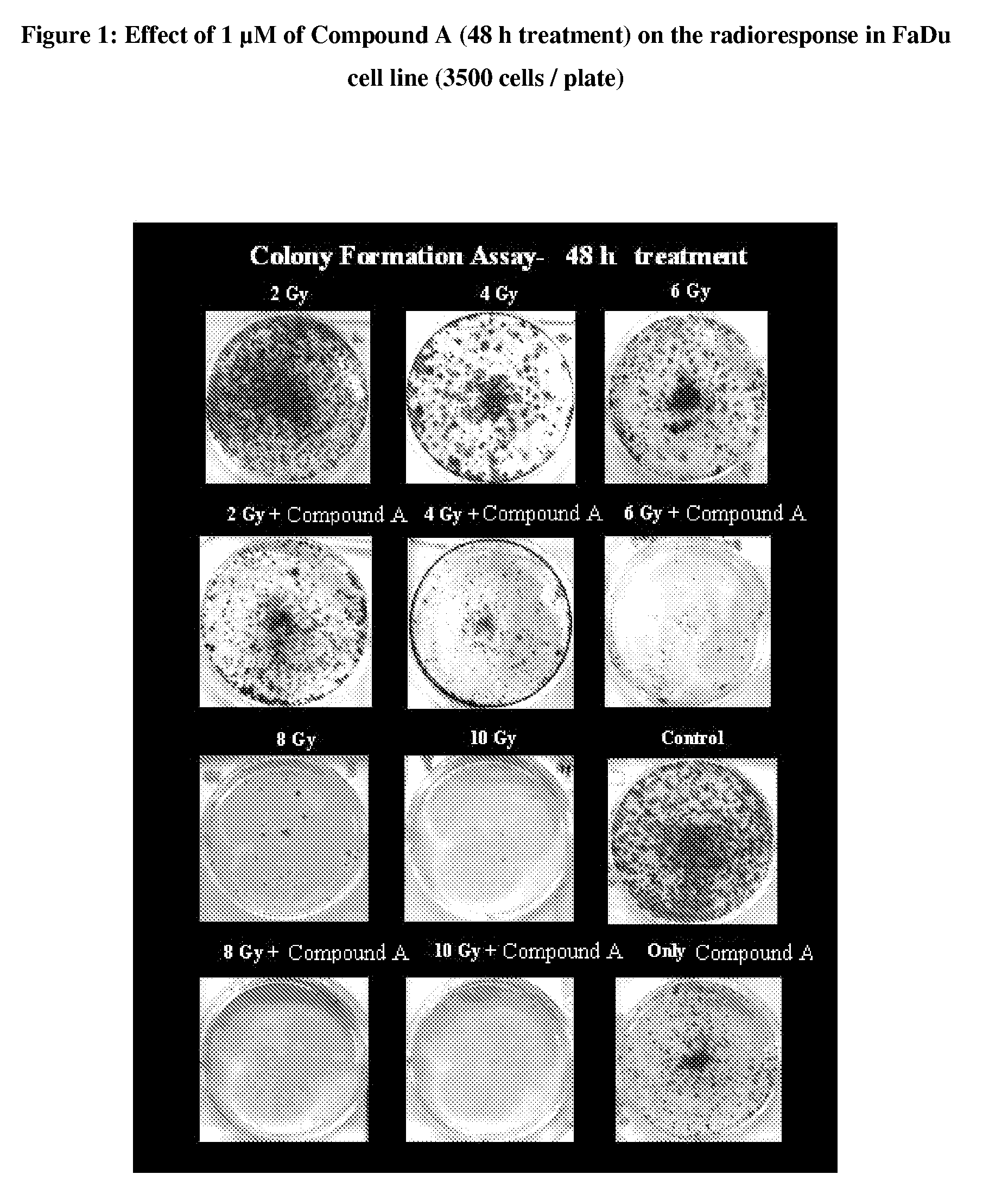
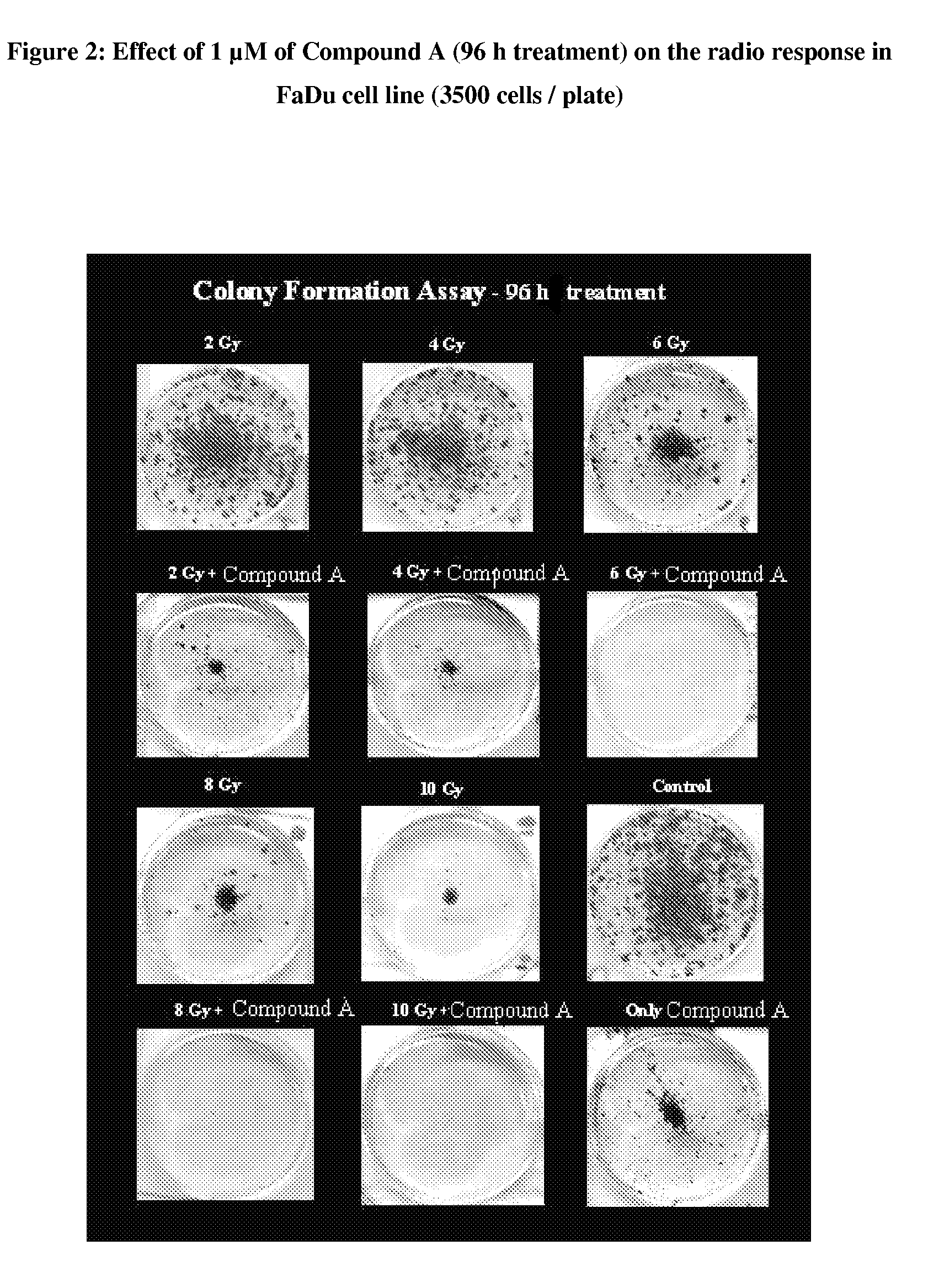
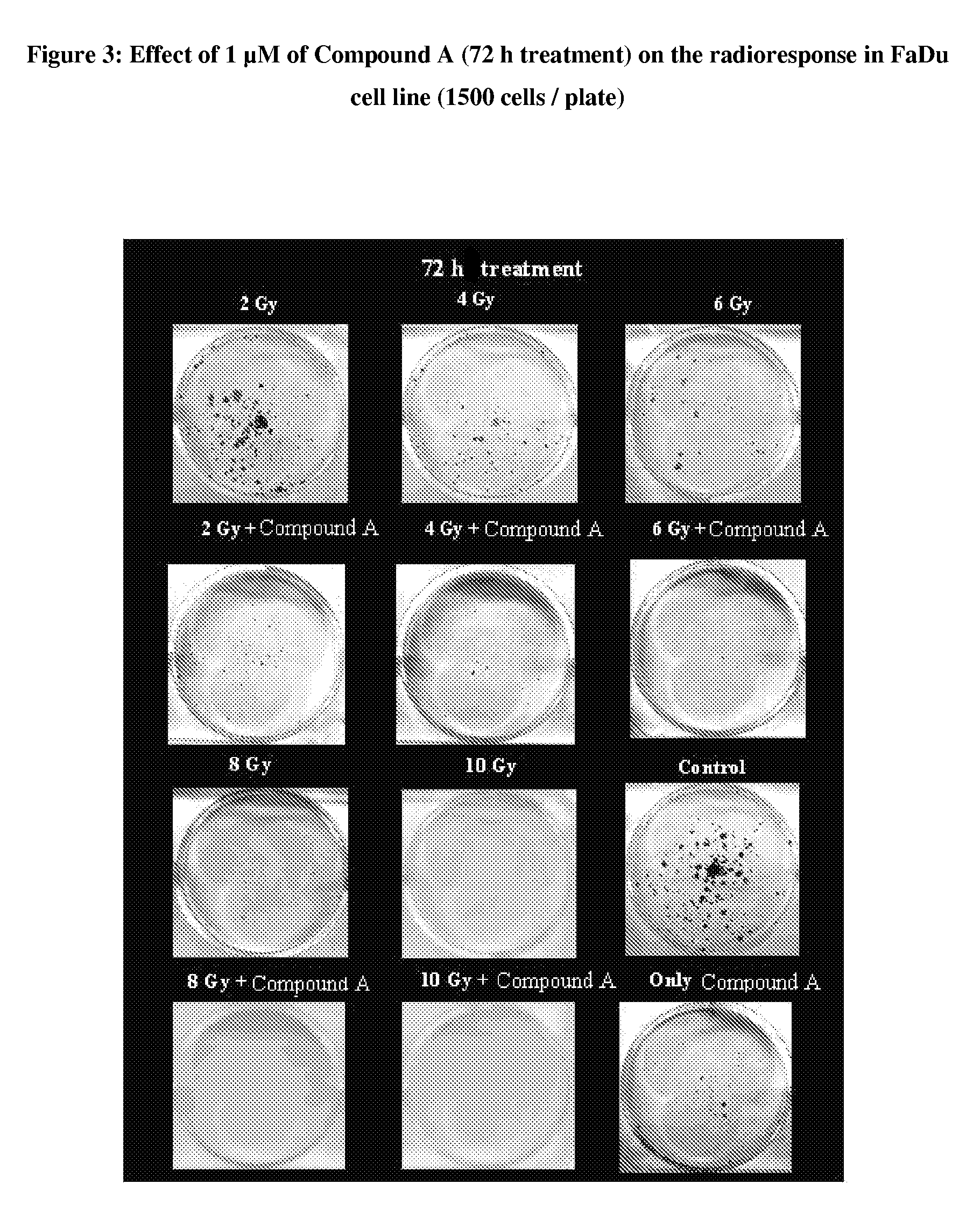
Pyrrolidine-substituted flavones as radio-sensitizers for use in the treatment of cancer
a technology of pyrrolidine and flavone, which is applied in the field of combination for the treatment of cancer, can solve the problems of insufficient oxygen, inability to further enhance the radiation effect, and inability to meet the needs of oxygen,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 1-Methyl-4-(2,4,6-trimethoxyphenyl)-1,2,3,6-tetrahydro pyridine
[0102]1-methyl-4-piperidone (340 g, 3.0 mol) was added slowly, to a solution of 1,3,5-trimethoxybenzene (500 g, 2.97 mol) in glacial acetic acid (600 mL), maintaining the temperature of the reaction mixture below 40° C. Conc. HCl (450 mL) was added over 20 min. The temperature was raised to 85-90° C. and the reaction mixture was stirred for 3.5 h. It was allowed to cool to 40° C., poured over crushed ice (4 kg) and stirred for 20 min. The precipitate of unreacted 1,3,5-trimethoxybenzene was filtered off. The filtrate was basified, below 10° C., to pH 11-12 using a 50% aqueous NaOH solution. The off white solid obtained was filtered, washed with water and dried to obtain the compound, 1-methyl-4-(2,4,6-trimethoxy-phenyl)-1,2,3,6-tetrahydropyridine.
[0103]Yield: 580 g (74%); mp: 112-114° C.; IR (KBr): 3045, 2900, 2837, 1600, 1585 cm−1; 1H NMR (CDCl3, 300 MHz): δ 6.15 (s, 2H), 5.55 (s, 1H), 3.85 (s, 3H), 3.75 ...
example 2
Preparation of (±)-trans-1-Methyl-4-(2,4,6-trimethoxyphenyl)-piperidin-3-ol
[0104]Boron trifluoride diethyl etherate (300 mL, 2.36 mol) was added slowly with stirring, under an atmosphere of nitrogen, at 0° C., to a solution of compound of example (1) (300 g, 1.14 mol) and NaBH4 (75 g, 1.97 mol) in dry THF (2.25 L). The temperature of the reaction mixture was slowly raised to 55° C. and stirred for 1.5 h. It was cooled to 30° C. Ice cold water (100 mL) was slowly added followed by acidification with conc. HCl (375 mL). The reaction mixture was stirred for 1 h. at 50-55° C. It was cooled to 30° C. and basified using 50% aqueous NaOH solution to pH 11-12. Hydrogen peroxide (30%, 225 mL) was added over 0.5 h. The reaction mixture was stirred at 55-60° C. for 1.5 h. It was cooled to 30° C. and sufficient water was added to dissolve the precipitated salts. The organic layer was separated and the aqueous portion extracted with ethyl acetate (2×1 L). The organic extracts were dried (anhydro...
example 3
Preparation of (±)-trans-Acetic acid-1-methyl-3-(2,4,6-tri ethoxyphenyl)-pyrrolidin-2-yl methyl ester
[0107]Methanesulfonyl chloride (30.27 mL, 44.8 g, 0.4 mol) was added drop wise to a cooled and stirred solution of compound of example (2) (100 g, 0.35 mol) and triethylamine (71.88 g, 0.7 mol) in dry THF (1.0 L). The reaction mixture was further stirred for 45 min. at 0° C. The precipitate of triethylamine HCl was filtered and washed with dry THF (2×100 mL). The filtrate was added dropwise to a refluxing suspension of sodium acetate (115 g, 1.40 mol) in 2-propanol (1.0 L). The reaction mixture was refluxed for a further 15 min., diluted with EtOAc (1.0 L) and salts were filtered. The mixture of salts was washed with EtOAc (2×100 mL). The combined filtrate was concentrated to give a gum. Water (50 mL) was added to the gum with stirring to obtain a solid which was filtered and dried to yield the compound, (±)-trans-acetic acid 1-methyl-3-(2,4,6-trimethoxy-phenyl)-pyrrolidin-2-yl methy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com