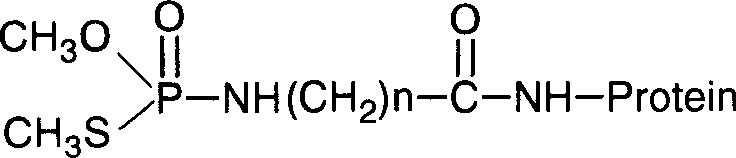Methamidophos half-antigen, artificial antigen and its preparing method
An artificial antigen, the technology of methamidophos, which is applied in the field of immunology, can solve the problems that do not involve the preparation of anti-methamidophos antibody, and do not mention the preparation method of methamidophos artificial antigen.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
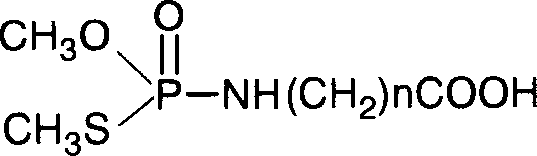
[0034] The preparation method of embodiment 1 methamidophos hapten (n=2)
[0035] 1) Dilute 1 part of O, S-dimethylphosphoryl thiochloride with 5 parts of anhydrous methanol;
[0036] 2) Add 1 part of β-alanine (NH 2 CH 2 CH 2 COOH) was dissolved in 5 parts of anhydrous methanol, and 2 to 3 parts of KOH were added;
[0037] 3) Add the solution of 2) dropwise to the solution of 1) in the stirring state, and continue to stir for 20 minutes after the addition, the product is adjusted to pH 2 with 1N HCl-chloroform, and the chloroform layer is passed over anhydrous MgSO 4 Dry and concentrate under reduced pressure to nearly dryness to obtain a yellow oil. Pass through a silica gel column (silica gel is a product of Qingdao Ocean Chemical Factory Branch), the eluent is methanol:petroleum ether=1:1, the product tubes are combined, and concentrated to dryness under reduced pressure to obtain the product O,S-dimethyl-N- (2-carboxyethyl) phosphorothioate. 1 H NMR: δ2.20(d, 3H, CH...
Embodiment 2
[0038] The preparation method of embodiment 2 methamidophos hapten (n=9)
[0039] 1) Dilute 1 part of O, S-dimethylphosphoryl thiochloride with 15 parts of absolute ethanol;
[0040] 2) 5 parts of 10-aminodecanoic acid (NH 2 (CH 2 ) 9 COOH) was dissolved in 15 parts of absolute ethanol, and 15 parts of NaOH were added;
[0041] 3) Add the solution of 2) dropwise to the solution of 1) in the stirring state, and continue to stir for 30 minutes after the addition, and adjust the pH of the product to 5 with 1N HCl-chloroform, take the chloroform layer and pass through anhydrous MgSO 4 Dry and concentrate under reduced pressure to nearly dryness to obtain a yellow oil. After passing through a silica gel column, the eluent was methanol:petroleum ether=3:1, the product tubes were combined, and concentrated to dryness under reduced pressure to obtain the product O, S-dimethyl-N-(9-carboxynonyl)thiophosphoramide .
Embodiment 3
[0042] Embodiment 3 active ester method prepares methamidophos artificial antigen
[0043] 80 micromole N,N'-dicyclohexylcarbodiimide (DCC) was dissolved in 1mL N,N-dimethylformamide (DMF), and the equivalent amount of the above hapten and N -in a DMF solution of hydroxysuccinimide (NHS), stirred overnight. The reaction supernatant was added dropwise into 5 mL of 10 mg / mL bovine serum albumin (BSA) carbonate buffer solution, stirred slowly overnight, and the product was transferred into a dialysis bag, and dialyzed against phosphate buffer at 4°C for 3 day, freeze-dried, subpackaged, and frozen. The product was made into a 1mg / mL solution with phosphate buffer solution for ultraviolet (200-400nm note: this is the wave path used for scanning, not measured with a single wavelength) spectrum scanning, and 1mg / mL BSA / phosphate buffer solution was used as In contrast, it was found that the ultraviolet spectrum of the product changed significantly compared with BSA, indicating tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com