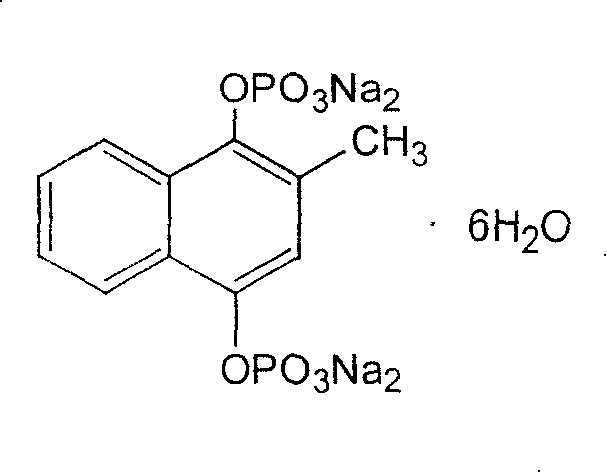
Sodium menadiol diphosphate ester and its pharmaceutical formulation
A technology of Naphthalene Hydroquinone Diphosphate Sodium and Naphthalene Hydroquinone Diphosphate, which is applied in the field of Menadione Hydroquinone Diphosphate Sodium Injection and its preparation, and can solve the problems of difficult long-term storage of preparations and poor chemical stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: menahydroquinone diphosphate sodium injection of 5mg specification
[0029] Menahydroquinone diphosphate sodium 5g, sodium metabisulfite 2.5g, sodium chloride 7.7g, water for injection was added to 1000ml. Measure 40% of the prepared water for injection, add the prescribed amount of sodium metabisulfite and sodium chloride and stir to dissolve, then add the main drug menahydroquinone diphosphate sodium, heat to dissolve, add 1g of activated carbon for needles at 60°C Insulate and absorb for 30 minutes, filter and decarburize when cooled to 45°C, add water for injection to 80% of the prepared amount, adjust the pH to 8.0 with 1mol / L sodium hydroxide, add water for injection to the full amount, measure the intermediate content and pH value, Then fine filter with 0.22μm microporous membrane, fill in ampoules after passing the test, 1ml each, fill with nitrogen, seal, steam sterilize, inspect, pack, and get ready.
Embodiment 2
[0030] Embodiment 2: menahydroquinone diphosphate sodium injection of 10mg specification
[0031] Menahydroquinone sodium diphosphate 10g, sodium metabisulfite 2.5g, sodium chloride 6.4g, water for injection was added to 1000ml. The preparation was prepared according to the same method as described in Example 1, and a 10 mg specification of menahydroquinone diphosphate sodium injection was prepared.
Embodiment 3
[0033] 3a) Except not containing sodium metabisulfite, the rest are the same as in Example 1, and the injection is prepared;
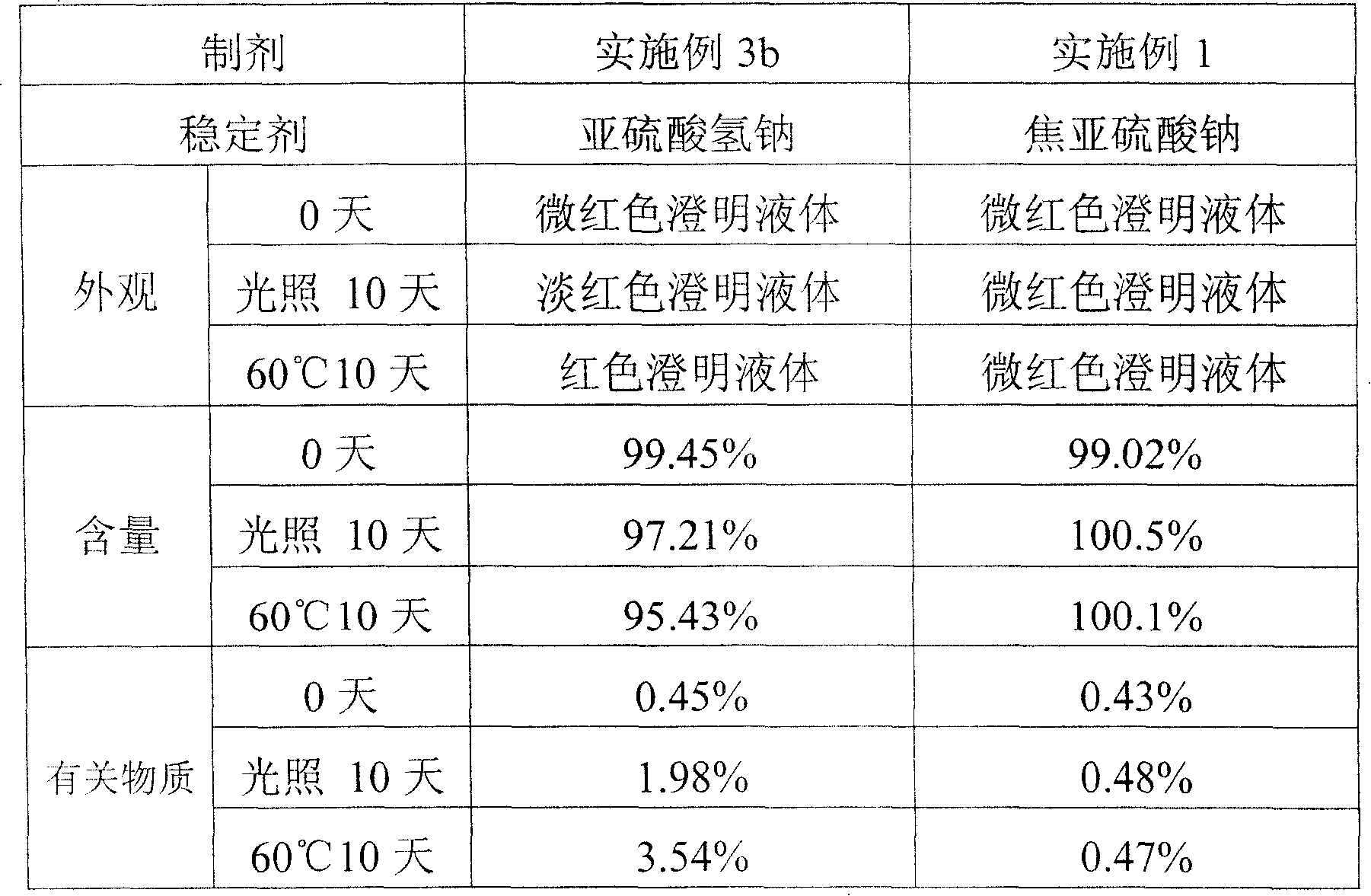
[0034] 3b) Except that the sodium metabisulfite was replaced with an equivalent amount of sodium bisulfite, the rest were the same as in Example 1, and the injection was prepared;
[0035] Strong light irradiation and high temperature stability tests were carried out. As a result, the 3a preparation without stabilizer had obvious discoloration and other phenomena, indicating that menahydroquinone diphosphate sodium was oxidatively degraded, and the stability of the preparation was poor, which did not meet the requirements of clinical medication. The 3b preparation containing sodium bisulfite also showed discoloration, and the content of sodium menahydroquinone diphosphate decreased. However, the preparation of Example 1 of the present invention using sodium pyrosulfite as a stabilizer has basically no discoloration phenomenon, and the content of sodium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com