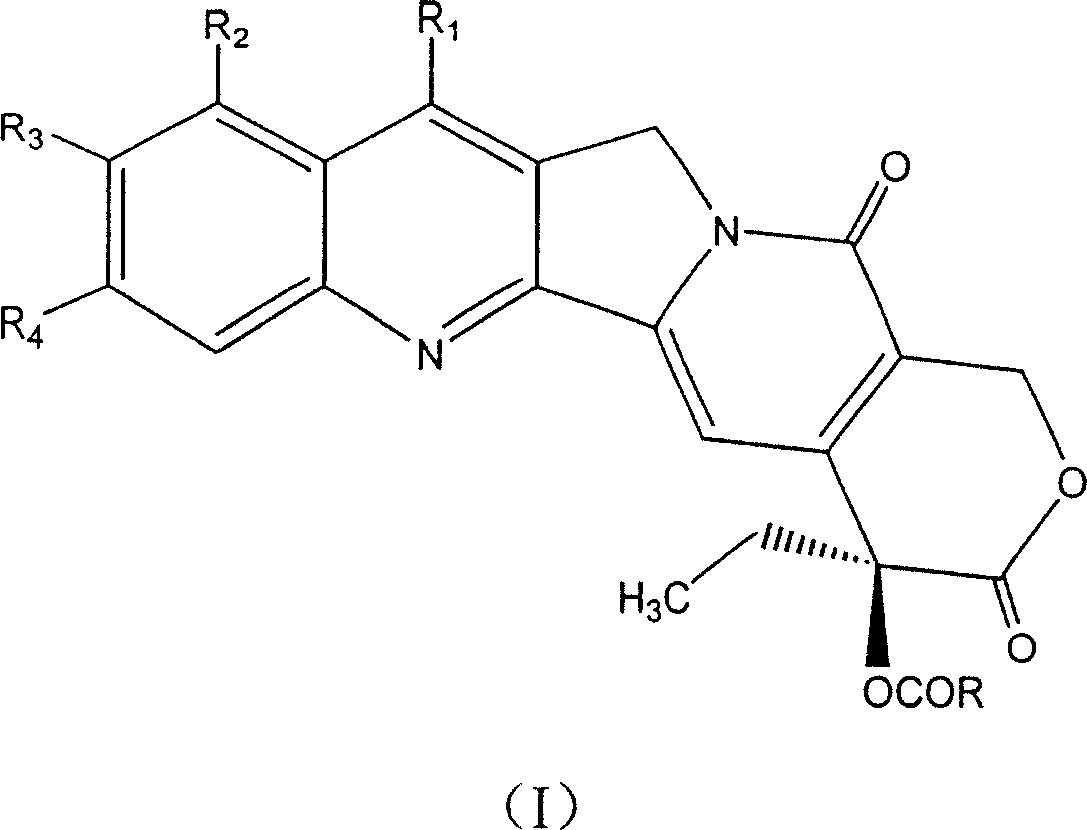
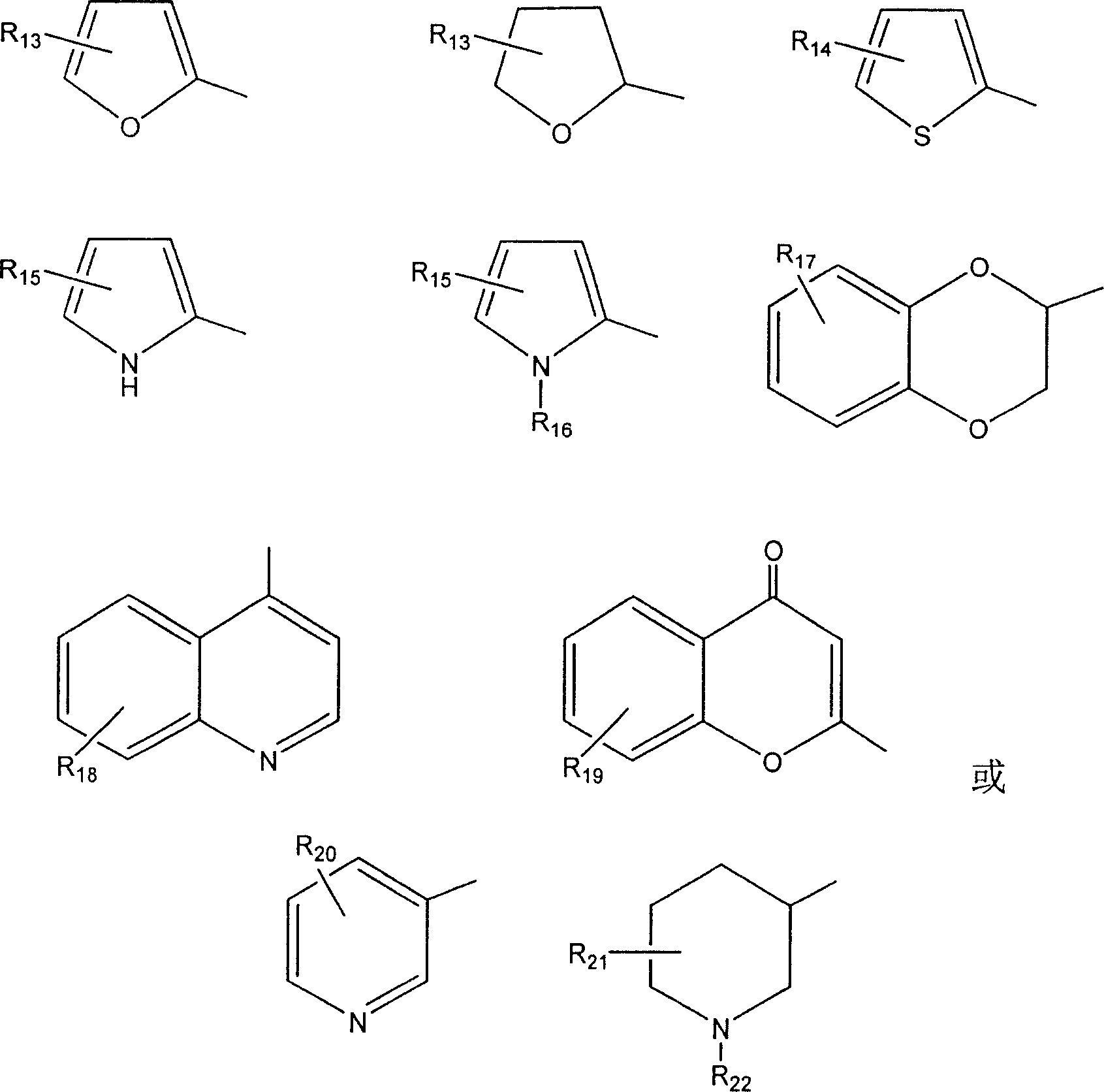
20-esterifiable camptothecine derivative and method for making same and pharmaceutical combination and uses
A compound, C1-C6 technology, applied in the field of anti-tumor drugs, can solve the problems of low anti-tumor activity and high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] 20S-camptothecin ester of embodiment 1.5-nitro-2-furancarboxylic acid (compound 1)
[0105] In a 10mL round bottom flask, add 10mg (0.028mmol) camptothecin, 9.0mg (0.057mmol) 5-nitro-2-furancarboxylic acid, 25mg (0.13mmol) 1-(3-dimethylaminopropyl)-3 - Ethylcarbimide hydrochloride (EDCI), 2 mg (0.019 mmol) 4-dimethylaminopyridine (DMAP) and 3 mL dichloromethane. After the reaction mixture was stirred at room temperature for 12 hours (clear), it was diluted with 20 mL of chloroform, and the chloroform layer was washed with water (15 mL), saturated sodium bicarbonate solution (15 mL) and saturated brine (15 mL), and dried over anhydrous magnesium sulfate . After removing magnesium sulfate by filtration, it was concentrated under reduced pressure. The residue was separated by silica gel column chromatography (eluent: chloroform-methanol 97:3) to obtain 12.0mg 20-O-5-nitro-2-furanoic acid camptothecin ester, yield: 85.0%, mp 228- 230°C.
[0106] 1 HNMR (CDCl 3 , 300MH...
Embodiment 2
[0107] 20S-camptothecin ester of embodiment 2.5-bromo-2-furancarboxylic acid (compound 2)
[0108] In a 10mL round bottom flask, add 10mg (0.028mmol) camptothecin, 10mg (0.052mmol) 5-bromo-2-furancarboxylic acid, 25mg (0.13mmol) 1-(3-dimethylaminopropyl)-3-ethane Ethylcarbimide hydrochloride (EDCI), 2mg (0.019mmol) 4-dimethylaminopyridine (DMAP) and 3mL dichloromethane. After the reaction mixture was stirred at room temperature for 36 hours, it was diluted with 20 mL of chloroform, and the chloroform layer was washed with water (15 mL), saturated sodium bicarbonate solution (15 mL) and saturated brine (15 mL), and dried over anhydrous magnesium sulfate. After removing magnesium sulfate by filtration, it was concentrated under reduced pressure. The residue was separated by silica gel column chromatography (eluent: chloroform-methanol 97:3) to obtain 3.0 mg 20-O-5-bromo-2-furanic acid camptothecin ester, yield: 20.0%, mp 203-206 ℃.
[0109] 1 HNMR (CDCl 3 , 300MHz): δ8.41(s...
Embodiment 3
[0110] 20S-7-ethylcamptothecin ester of embodiment 3.5-nitro-2-furancarboxylic acid (compound 3)
[0111] In a 10mL round bottom flask, add 10mg (0.027mmol) 7-ethylcamptothecin, 8.0mg (0.052mmol) 5-nitro-2-furancarboxylic acid, 25mg (0.13mmol) 1-(3-dimethylaminopropyl Dimethyl)-3-ethylcarbimide hydrochloride (EDCI), 2mg (0.019mmol) 4-dimethylaminopyridine (DMAP) and 3mL dichloromethane. After the reaction mixture was stirred at room temperature for 12 hours (clear), it was diluted with 20 mL of chloroform, and the chloroform layer was washed with water (15 mL), saturated sodium bicarbonate solution (15 mL) and saturated brine (15 mL), and dried over anhydrous magnesium sulfate . After removing magnesium sulfate by filtration, it was concentrated under reduced pressure. The residue was separated by silica gel column chromatography (eluent: chloroform-methanol 97:3) to obtain 11.2 mg of 20-O-5-nitro-2-furancarboxylic acid-7-ethylcamptothecin ester, yield: 81.8 %, mp 297-299° ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com