Virus in deactivated bioproduct and method of removing toxin in bacteria
A technology of bacterial endotoxin and biological products, applied in the direction of inactivation/attenuation, etc., can solve the problems of inability to use, large influence of cytokine biological activity, expensive equipment, etc., to maintain biological activity, good inactivation effect, and ensure safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
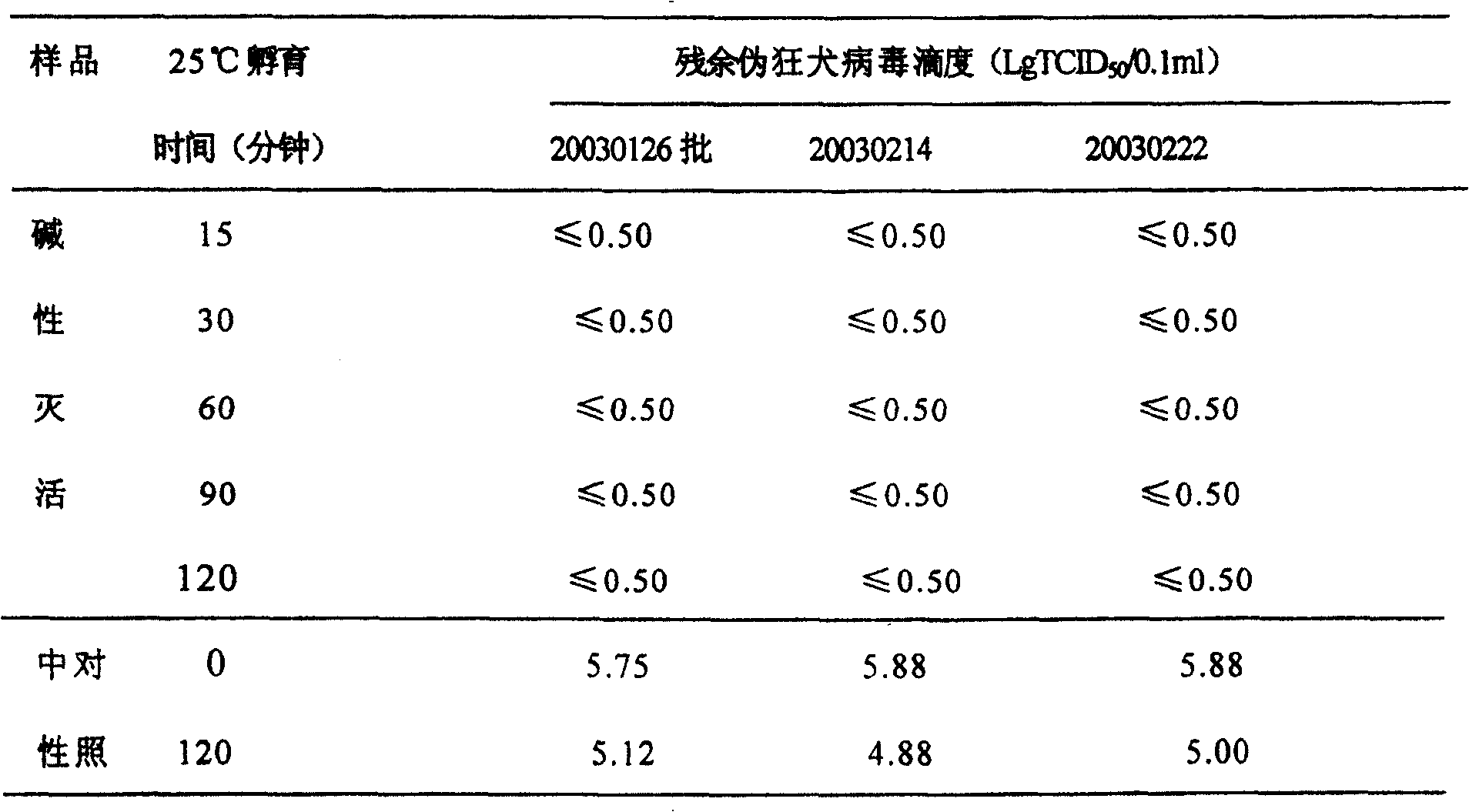
[0019] Embodiment 1 Inactivation test of human nerve growth factor injection intermediate product pseudorabies virus
[0020] Indicating virus: pseudorabies virus, adding titer is 8.00LgTCID 50 / ml
[0021] Cells for culture: PK-15 cells.
[0022] 1. Virus inactivation according to the steps:
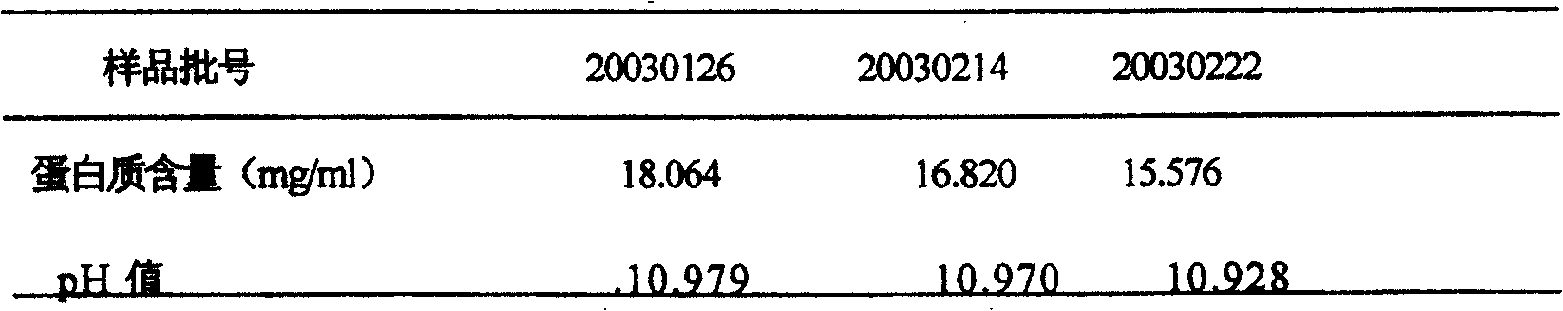
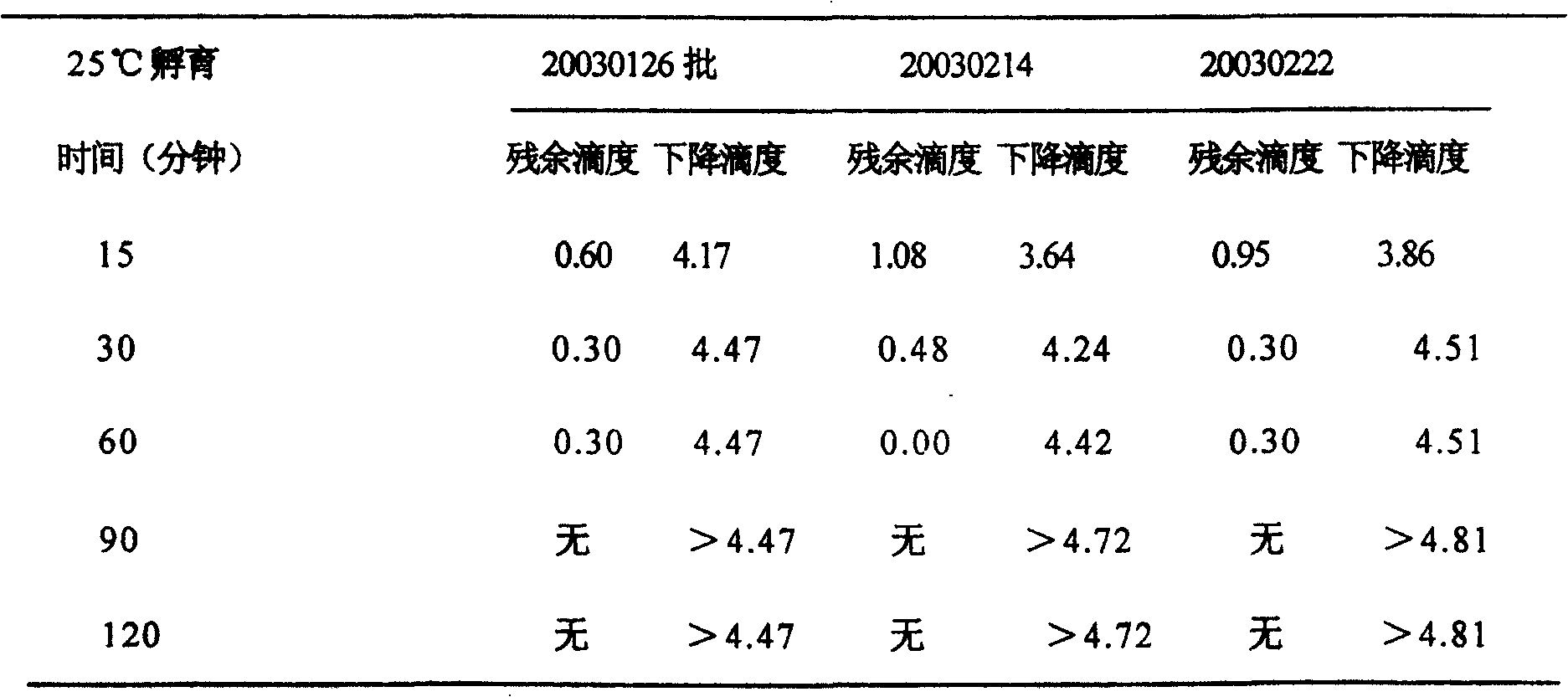
[0023] Take 45ml of each of the three batches of samples, add 5ml of pseudorabies virus respectively, and add endotoxin at the same time, the concentration is 4EU / ml, mix well, adjust the pH value to 12±0.1 with 4mol / L NaOH, and equally distribute to 5 centrifuge tubes , 8ml / tube, sealed, incubated in an incubator at 25°C±1°C, sampled at 15, 30, 60, 90, and 120 minutes, and immediately added 3mol / L HCl to adjust the pH to neutral.
[0024] The above treated samples were centrifuged at 4°C (4000rpm) for 30 minutes, and the supernatant was collected and filtered into small centrifuge tubes, 1.2ml / tube, 4 tubes / sampling point, and frozen at -70°C for detection.
[0025] 2. Neutral cont...
Embodiment 2
[0037] Carry out the inactivation test of 2 human nerve growth factor injection intermediate product Sindbis virus
[0038] Indicator virus: Sindbis, titer is 7.34LgTCID 50 / ml.
[0039] Cells used for culture: BHK-21, titration method: 6-well plate plaque method.
[0040] Follow the steps for virus inactivation:
[0041] Take 20ml of each of the three batches of samples and adjust to neutral with 3mol / L HCl.
[0042] Take 45ml of each of the three batches of samples, add 5ml of Sindbis virus, adjust the pH value to 12±0.1 with 4mol / L NaOH, divide each batch into 5 centrifuge tubes with 8ml / tube, incubate at 25°C±1°C, and incubate at 15 , 30, 60, 90, and 120 minutes later, samples were taken, and 80 μl of 3mol / L HCl was added immediately to adjust the pH value to neutral.
[0043] The above inactivated samples were centrifuged (4000rpm) for 30 minutes, filtered with a 0.22 μm membrane, and the supernatant was divided into small centrifuge tubes, 4 tubes per batch, and froz...
Embodiment 3
[0049] Embodiment 3 Inactivation test of human nerve growth factor injection intermediate product EMCV virus
[0050] Indicator virus: EMCV, titer: 7.50LgTCID 50 / 0.1ml.
[0051] Cells used for culture: Vero, titration method: 96-well micro-lesion method.
[0052] Virus inactivation: with embodiment 2 (except that the added virus Sindbis is changed into EMCV, all the other are identical with embodiment 2).
[0053] Virus detection method: Vero cell 96-well micro-lesion method was used.
[0054] The results of virus titration showed that three batches of human nerve growth factor were added to the indicator virus EMCV, and the virus detection results after being inactivated by the method of the present invention are shown in Table 3.
[0055] table 3
[0056]
[0057] Note: The titer unit is LgTCID 50 / 0.1ml.
[0058] As seen from Table 3, this method can inactivate this virus titer as:
[0059] 20030126 batches > 4.250LgTCID 50 / 0.1ml;
[0060] 20030214 batches > 4.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com