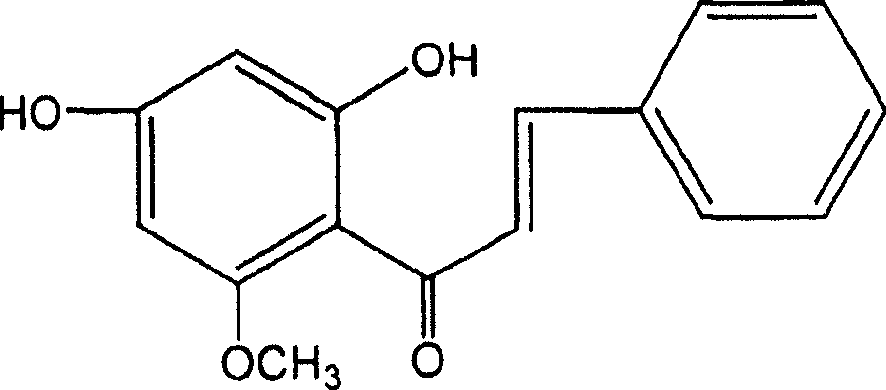
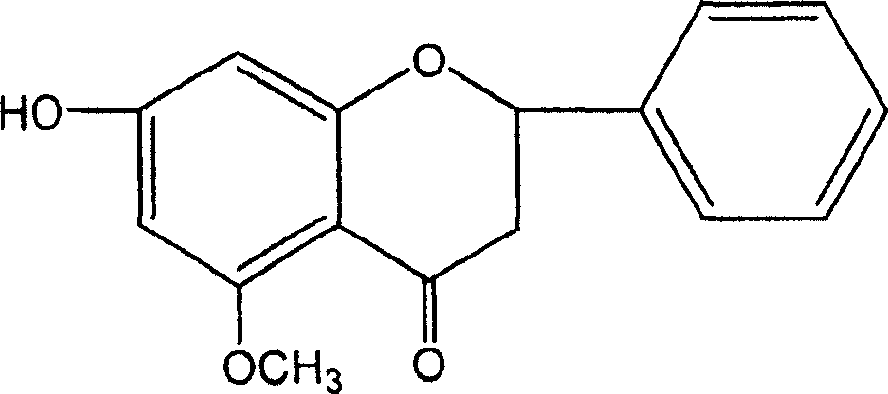
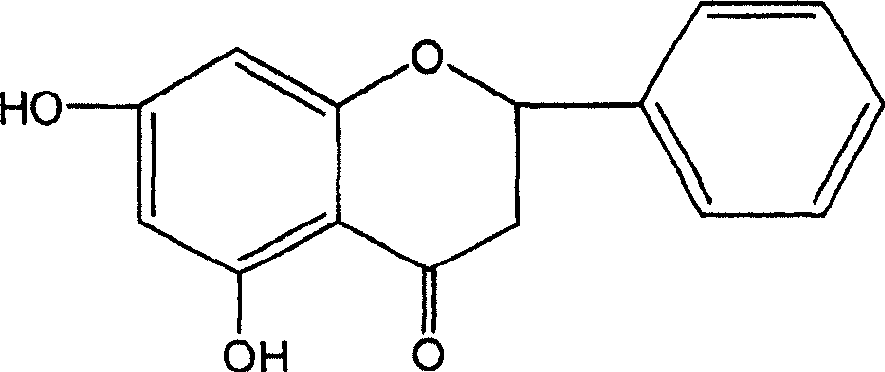
Application of katsumada galangal seed or its substitute extracts or its inclusive compound in pharmacy
An extract, the technology of cardamom, applied in the field of application of cardamom or its substitute extract or its contained compounds in pharmaceuticals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Preparation of Cardamom officinalis Extract and Separation and Purification of Four Monomer Compounds
[0047] Take 18kg of cardamom medicinal material, pulverize it, reflux extract with 70-90% industrial ethanol for three times, filter the extract while it is hot, combine the filtrates, recover the solvent under reduced pressure, and obtain 1300 g of dry extract (yield: 7.2%), which is cardamom Extract.
[0048] After the extract was diluted with water to form a suspension, it was extracted three times with petroleum ether, ether, ethyl acetate and n-butanol respectively to obtain petroleum ether, ether, ethyl acetate, n-butanol and aqueous solution. The fractions of petroleum ether, diethyl ether and ethyl acetate were subjected to silica gel column chromatography and eluted with petroleum ether-ethyl acetate gradient to obtain compounds CDK1, CDK2, CDK3 and CDK4.
Embodiment 2
[0049] Embodiment 2: drug to cytotoxicity test
[0050] 1. Experimental materials
[0051] 1) Compounds: CDK-1, CDK-2, CDK-3 and CDK-4 are monomeric compounds extracted and isolated from the medicinal material Cardamom, with a purity greater than 95%. Yellow or white powder. CDK-1, CDK-2, CDK-3 and CDK-4 were made into 50 mg / ml mother solution with DMSO, and diluted with culture medium to the required concentration before use. Positive control drug Ribavirin (RBV) is produced by Hubei Qianjiang Pharmaceutical Factory, white powder, made into 10mg / ml mother solution with double distilled water (DDW), positive control drug Acyclovir (ACV) is Hubei Qianjiang Pharmaceutical Factory production, white powder, mixed with double distilled water (DDW) to make 1mg / ml mother solution.
[0052] 2) Cells: MDCK cells; Vero cells, subcultured in our laboratory.
[0053] 3) Reagents, laboratory supplies and instruments:
[0054] Eagles MEM dry powder, glutamine are all products of GIBCO,...
Embodiment 3
[0068] Embodiment 3: The inhibitory test of medicine to influenza virus
[0069] 1) Use the same relevant experimental materials and cell culture methods as in Example 2 to carry out the experiment. Influenza virus type A / Jifang / 15 / 90(H3N2), A / Yuefang / 243 / 72(H3N2), A / Jingfang / 262 / 95(H1N1); Influenza virus type B / Jifang / 13 / 97.
[0070] 2) Experimental method:
[0071] Inoculate 96-well cell culture plate with 200,000-300,000 MDCK cells per milliliter, 0.1 ml per well, 37°C, 5% CO 2 Cultivate for 24 hours, discard the culture medium, add an appropriate amount of virus, after 2 hours of adsorption, discard the virus solution, add the influenza upper layer containing non-toxic concentrations of CDK-1, CDK-2, CDK-3 and CDK-4, and set up 5 dilutions in total degree, 3 wells per concentration, 37°C, 5% CO 2 to cultivate. When the lesions of the virus control group reach 4, observe the results, 4 is completely destroyed; 3 is 75%; 2 is 50%; 1 is 25%; 0 is no lesion. At the same...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com